Lilly’s ZEPBOUND Clears Hurdle in Sleep Apnea Treatment
Jan 06, 2025
Eli Lilly’s blockbuster weight loss GLP-1 drug ZEPBOUND has received FDA approval to treat obstructive sleep apnea in adults with obesity, alongside diet and exercise. This marks the first FDA-approved medication for obstructive sleep apnea.
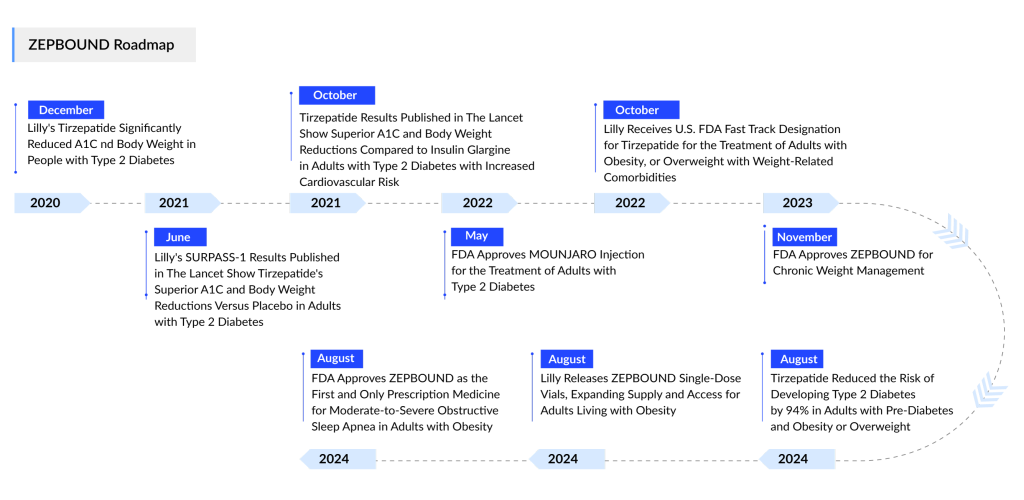
The approval adds a second indication for ZEPBOUND, following its initial authorization for obesity in November 2023. The drug’s active ingredient, tirzepatide, is also marketed at lower doses for Type 2 diabetes under the brand name MOUNJARO. The drug functions as a dual agonist of the GLP-1 and GIP receptors, which regulate insulin secretion and appetite suppression.
In addition to obesity and sleep apnea, Lilly is exploring its tirzepatide franchise for other cardiometabolic conditions, including heart failure, prediabetes, and metabolic dysfunction-associated steatohepatitis (MASH).
Downloads
Article in PDF
Recent Articles
- Obesity: A Worldwide Pandemic with Advancing Management Options
- Sleep Aids Market: Examining Cutting-Edge Technologies and Major Breakthroughs
- FDA Grants Priority Review to Merck’s Application for KEYTRUDA Plus Padcev; Roche and Carmot Ther...
- Beyond Supportive Care: How New Drugs are Shaping Metabolic-associated Steatohepatitis (MASH) Tre...
- Eli Lilly to acquire Dermira, Boehringer buys Enleofen’s IL-11 platform, Biogen acquires Pfizer’s...
“OSA is frequently dismissed as mere ‘snoring,’ but it’s much more serious,” stated Julie Flygare, J.D., president and CEO of Project Sleep. “Recognizing the symptoms of OSA and knowing that effective treatments exist, including innovative options like ZEPBOUND, is crucial. We aim to inspire deeper discussions between patients and healthcare professionals, ultimately driving improved health outcomes.”
The recent approval targets adults with moderate-to-severe obstructive sleep apnea and obesity, intended for use alongside diet and exercise. The Phase III SURMOUNT-OSA trial demonstrated that ZEPBOUND was five times more effective in reducing breathing disruptions in adults not using positive airway pressure (PAP) therapy—the standard device for managing sleep apnea. After 52 weeks, 50% of participants on the drug combined with a PAP machine achieved either remission or mild, non-symptomatic OSA, compared to 14% on a placebo. The drug alone also showed strong results, with 42% experiencing remission or mild OSA after one year.
In the US, an estimated 30–90 million people have OSA, though only 6 million are diagnosed. In the assessment done by DelveInsight, the estimated obstructive sleep apnea diagnosed prevalent cases in 2023 was highest in the US among the 7MM with 54% cases alone, accounting for nearly 13.7 million cases.
The condition is linked to higher risks of heart disease, stroke, and workplace errors. ZEPBOUND’s achievement in addressing sleep apnea marks the culmination of a remarkable year for the tirzepatide franchise, which has played a pivotal role in Lilly’s recent growth and expansion initiatives.
“Currently, many individuals with OSA remain undiagnosed and untreated, exposing millions to significant health risks,” stated Patrik Jonsson, executive vice president and president of Lilly Cardiometabolic Health and Lilly USA. “ZEPBOUND is the first medication to offer substantial improvements for moderate-to-severe OSA while also supporting long-term weight loss in adults with obesity. Nearly half of the clinical trial participants experienced such remarkable progress that their OSA symptoms were entirely alleviated, representing a major advancement in addressing the burden of this condition and its related health challenges.”
In its primary indication for weight loss, ZEPBOUND recently outperformed Novo Nordisk’s WEGOVY by 47% in the head-to-head SURMOUNT-5 trial. Patients on ZEPBOUND experienced an average weight reduction of 20.2% over 72 weeks, compared to 13.7% for those on Wegovy.
Earlier, in November 2024, Lilly shared impressive findings from the three-year SURMOUNT-1 trial, which demonstrated that tirzepatide reduced the risk of progression to diabetes by 94% compared to placebo in overweight or obese adults with prediabetes.
Sales for Eli Lilly’s tirzepatide products, MOUNJARO and ZEPBOUND, totaled $4.37 billion in Q3 2024, slightly below analyst expectations due to drug shortages. This allowed compounding pharmacies to produce cheaper, unbranded alternatives. However, on December 19, the FDA announced the resolution of the shortage, giving pharmacies previously approved for compounding 60 to 90 days to cease production of these versions.
Eli Lilly is exploring additional indications for its metabolic blockbuster, tirzepatide. In June 2024, the company shared detailed results demonstrating the drug’s potential in treating MASH, a fatty liver disease formerly known as NASH.
The mid-stage SYNERGY-NASH trial revealed that fibrosis improved by at least one stage without worsening MASH in 54.9% of patients on a 5 mg dose of tirzepatide, 51.3% on a 10 mg dose, and 51% on a 15 mg dose. In contrast, only 29.7% of patients in the placebo group experienced similar fibrosis improvement.
In addition to ZEPBOUND, Eli Lilly is evaluating another GLP-1 receptor Retatrutide in the late stage of development for OSA. Retatrutide (LY3437943), a biologic drug under development by Eli Lilly and Company, acts as a triagonist targeting the gastric inhibitory polypeptide (GIP) receptor, the glucagon-like peptide-1 (GLP-1) receptor, and the glucagon receptor. It is being studied for its potential to treat obesity, osteoarthritis, and obstructive sleep apnea, with a planned approach for simultaneous regulatory submissions. The company is currently enrolling participants for Phase III clinical trials to evaluate the efficacy and safety of LY3437943. The launch of Retatrutide will further propel the GLP-1 market.
GLP-1 drugs are gaining significant traction in the obesity market. Since Novo Nordisk’s semaglutide received approval for a cardiovascular indication in March 2024, this class of drugs is being studied for a wide range of conditions, including chronic kidney disease, metabolic dysfunction-associated steatohepatitis, Alzheimer’s disease, cancers, and even addiction.
Historically, Medicare has excluded obesity medications from coverage. However, approvals for additional indications have broadened access to these treatments for millions of Americans. The Biden administration has proposed a new rule to classify obesity medications as treatments for “chronic diseases” instead of weight loss aids. This shift could substantially lower out-of-pocket expenses for the drugs, which currently exceed $1,000 per month, benefiting 3.4 million Medicare beneficiaries and an additional 4 million Medicaid enrollees.

Downloads
Article in PDF
Recent Articles
- Sleep Aids Market: Examining Cutting-Edge Technologies and Major Breakthroughs
- Apollomics raises; Pear Therapeutics raises $64M; Lilly to acquire Loxo
- Commercial
- ATS 2023 Updates: AD109’s Potential as the First Oral Medication for Obstructive Sleep Apne...
- Lilly moves ahead; Authorities raid; Bristol-Myers, AZ in legal; PhRMA expected to weed out