ZORYVE for Atopic Dermatitis Treatment: Is the Breakthrough We’ve Been Waiting for?
Jul 19, 2024
After a few days of investor anxiety, Arcutis Biotherapeutics announced that the FDA has approved ZORYVE for a new indication: atopic dermatitis. The FDA was scheduled to announce its decision on ZORYVE (roflumilast) for this new use on the 7th of this month, but the date passed without news. In an uncommon move to calm shareholders’ concerns, Arcutis released a statement indicating no new information had been requested and that they anticipated receiving a letter from the regulator shortly.
Later on July 09, confirmation was finally received: ZORYVE had been approved as a new, once-daily treatment for mild to moderate atopic dermatitis in adults and children aged six and older. ZORYVE (roflumilast) cream, a topical medication, functions by inhibiting phosphodiesterase-4 (PDE4), an enzyme within cells that promotes the production of inflammatory substances while reducing anti-inflammatory substances.
By entering the atopic dermatitis treatment market, Arcutis opens access to ~72 million individuals in the 7MM as of 2023, as reported by DelveInsight. Atopic dermatitis is more prevalent in children than in adults across the 7MM. Approximately 5–10% of patients with mild atopic dermatitis progress to moderate or severe cases within a year.
Downloads
Article in PDF
Recent Articles
- Assessment of Key Products that Got FDA Approval in Second Half (H2) of 2021
- Atopic dermatitis market and a Dupilumab dominance
- Atopic dermatitis market: Increasing prevalence and topical treatments
- Aslan Pharma – IQVIA collaboration; Reata’s kidney drug bardoxolone; Alzheimer’s Dise...
- 6 Emerging Treg Cell-based Therapies Shaping the Future of Immunotherapy
The regulatory approval is supported by data from three Phase III trials, including INTEGUMENT-1 and INTEGUMENT-2. Both of these late-stage studies achieved their primary goal of treatment success, defined as a clear or almost clear score on the validated Investigator Global Assessment-Atopic Dermatitis scale, with a two-grade improvement after four weeks.
Additionally, the INTEGUMENT studies demonstrated that ZORYVE significantly reduces itching and eczema severity in patients, with noticeable effects as early as the first week. ZORYVE was also found to be safe, with low overall rates of treatment-emergent adverse events. Most side effects were mild or moderate in severity, with the most common being headache, nausea, and application-site pain.
Arcutis plans to widely distribute ZORYVE cream 0.15% through major wholesalers and dermatology pharmacies by the end of July. The company is committed to ensuring consistent access to the ZORYVE product line with a straightforward copay and fulfillment process. The ZORYVE Direct Program aids patients in obtaining their prescribed Arcutis medication, helping them navigate the payer system and adhere to their treatment. This program also includes the ZORYVE Direct Savings Card Program, which can lower out-of-pocket expenses for eligible patients with commercial insurance. Additionally, Arcutis will continue to provide the Arcutis Cares patient assistance program, offering ZORYVE at no cost to financially eligible patients who are uninsured or underinsured.
The approval of ZORYVE cream 0.15% for the atopic dermatitis indication marks Arcutis’ third FDA approval of a commercial product in the last two years. Initially, a 0.3% formulation of the cream was approved and launched in 2022 for treating plaque psoriasis topically in patients aged 12 years and older.
Last year, the FDA expanded the drug’s label to include treatment for psoriasis in patients aged 6-11 years. Psoriasis is prevalent worldwide with a global prevalence of 1% to 8%. It begins in childhood in approximately one-third of cases. In 2023, the total number of diagnosed prevalent cases of psoriasis in the US was estimated to be around 8 million, as per DelveInsight.
In 2023, the FDA approved Arcutis’ NDA for ZORYVE topical foam 0.3% for treating seborrheic dermatitis in patients aged nine years and older. Following this approval, Arcutis’ ZORYVE foam became the first FDA-approved drug in over two decades to treat seborrheic dermatitis with a new mechanism of action.
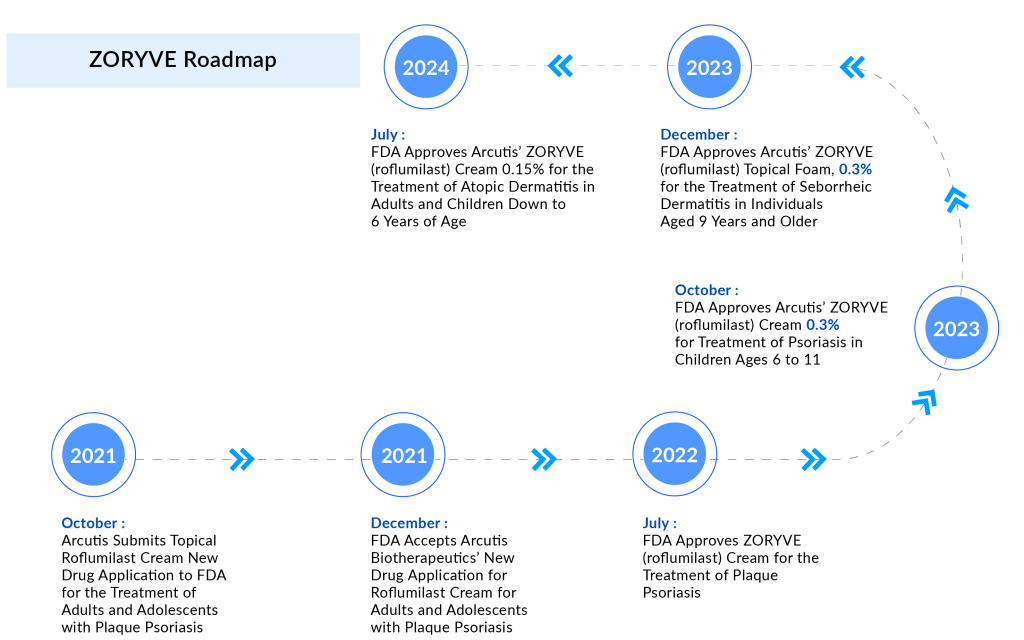
ZORYVE has experienced rapid adoption since its introduction, driven by increasing demand for its units. Arcutis reported $15 million in sales from ZORYVE cream 0.3% during the first quarter of 2024, marking a remarkable 675% growth compared to the same quarter last year. Revenue from sales of ZORYVE topical foam 0.3% reached $6.5 million, showing a sequential increase of 59%.
The company is currently assessing roflumilast cream for atopic dermatitis in children aged two to five years at a reduced concentration of 0.05%. Additionally, Arcutis has finished its clinical development program for ZORYVE foam 0.3%, intended for treating scalp and body psoriasis. It intends to file a supplemental New Drug Application (sNDA) for this expanded use by the third quarter of 2024. If approved, these new indications could significantly enhance revenue opportunities.
It faces competition in its category from non-steroid medications such as Incyte’s JAK inhibitor OPZELURA (ruxolitinib), which was approved for atopic dermatitis treatment in 2021. However, OPZELURA requires twice-daily dosing and has a more restrictive label that limits its use to short-term and non-continuous chronic treatment in adults and children aged 12 and older. OPZELURA achieved sales of slightly over $100 million last year across atopic dermatitis and vitiligo, its second indicated condition.
Additional potential competitors include Dermavant’s TAMA drug VTAMA (tapinarof), which is already approved for psoriasis and is awaiting an FDA decision later this year for atopic dermatitis treatment in patients aged two and older.
Tapinarof represents a novel aryl hydrocarbon receptor agonist currently in development. It is designed for topical application once daily, free from steroids, aiming to provide a pleasant cosmetic experience. At present, Dermavant is conducting two Phase III trials (NCT05014568, NCT05032859) targeting both pediatric and adult patients with atopic dermatitis. Additionally, there is an ongoing Phase III long-term extension trial (NCT05142774) for patients who completed treatment in either of the two pivotal studies in the United States. In March 2023, positive top-line results from the Phase III ADORING 2 trial were announced, demonstrating the successful achievement of all primary and secondary endpoints.
Another contender is Leo Pharma’s JAK inhibitor CORECTIM (delgocitinib), approved for atopic dermatitis in Japan and currently under review for chronic hand eczema in the US and Europe. Moreover, the current atopic dermatitis pipeline consists of a great deal of drugs. Rocatinlimab (KHK4083/AMG 451) (Amgen/Kyowa Kirin), KAPRUVIA/KORSUVA (difelikefalin) (Cara Therapeutics), and some others are the most highlighted drugs of this indication which can give a tough fight to ZORYVE.
Rocatinlimab (KHK4083/AMG 451) is a human monoclonal antibody targeting OX40, designed to inhibit pathogenic T cells that express OX40, thereby reducing systemic and local inflammatory responses. Effector T cells expressing OX40 are implicated in the pathophysiology of conditions like atopic dermatitis. Currently, in Phase III clinical trials, Rocatinlimab is being developed by Amgen and Kyowa Kirin under a partnership announced in June 2021. Their collaboration aims to develop and commercialize this antibody for treating atopic dermatitis, and potentially other autoimmune diseases.
Amgen and Kyowa Kirin have recently started enrolling participants for a large-scale global Phase III initiative known as the ROCKET program. This program seeks to evaluate the safety and efficacy of rocatinlimab in a wide-ranging group of individuals with moderate-to-severe atopic dermatitis, who experience significant disease challenges. The companies have outlined an estimated timeline for potential approval of this atopic dermatitis treatment around 2026/2027.
KAPRUVIA/KORSUVA (difelikefalin) is a new oral compound developed by Cara Therapeutics. It targets kappa opioid receptors located outside the central nervous system, rather than mu receptors. This approach utilizes unique chemical properties to restrict its entry into the CNS, directing its effects towards kappa receptors in the peripheral nervous system. Unlike drugs that activate mu receptors in the CNS, potentially causing side effects such as psychiatric issues, difelikefalin aims to mitigate these risks by focusing on kappa receptors.
Cara Therapeutics has completed Phase II trials involving adults with atopic dermatitis and moderate to severe itching. Currently, difelikefalin is in Phase III trials (NCT05387707; KIND-1) to evaluate its safety and effectiveness as an additional therapy with topical corticosteroids for moderate-to-severe itching in adults with atopic dermatitis. The company expects to announce the top-line results of the KIND 1 trial by the first half of 2025.
The anticipated launch of these emerging therapies presents a significant challenge to ZORYVE in the atopic dermatitis treatment market, particularly in terms of revenue. These novel treatments bring innovative mechanisms of action and promising clinical outcomes, potentially reshaping treatment paradigms and patient outcomes. As they enter the atopic dermatitis treatment market, they introduce competitive pressures that may divert market share from established treatments like ZORYVE. Their advancements in efficacy, safety profiles, or patient convenience could appeal to both patients and healthcare providers, thereby posing a formidable competitive landscape for ZORYVE and necessitating strategic adaptations to maintain its market position and revenue streams.

Downloads
Article in PDF
Recent Articles
- GSK’s RSV Vaccine Clears Phase III Test in Adults; Roche’s Tecentriq for Adjuvant NSCLC; Owkin Ba...
- 5 Promising Exosome-based Therapies Paving the Way for Personalized Medicine
- Atopic dermatitis market: Increasing prevalence and topical treatments
- Immutep’ First-Line Treatment Positive Outcomes; Pfizer’s Once-Daily Oral GLP-1 Agonist Danuglipr...
- Immunocore’s Kimmtrak; Samsung Acquires Biogen’s Biosimilar Unit; Novavax’s COVID-19 Vaccin...



