acinetobacter infections pipeline insight
DelveInsight’s, “Acinetobacter Infections - Pipeline Insight, 2025” report provides comprehensive insights about 8+ companies and 10+ pipeline drugs in Acinetobacter Infections pipeline landscape. It covers the pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Geography Covered
- Global coverage
Acinetobacter Infections: Understanding
Acinetobacter Infections: Overview
Acinetobacter, particularly *A. baumannii*, is a gram-negative, aerobic, non-fermentative, oxidase-negative, and nonmotile bacterium commonly found in soil and water. While it generally has low virulence, it can cause infections in immunocompromised or neutropenic patients, particularly in healthcare settings. Acinetobacter is frequently cultured from urine, respiratory secretions, saliva, and open wounds, and it can also colonize intravenous fluids and irrigation solutions. Infections are often a result of nosocomial spread and colonization rather than new infections. Key risk factors include prolonged stays in intensive care units, mechanical ventilation, prolonged antibiotic use, central venous catheter use, and hemodialysis. Acinetobacter infections tend to occur in outbreaks, especially in ventilated patients, where its presence in respiratory secretions often indicates colonization rather than active infection.
Acinetobacter, primarily a nosocomial pathogen, is commonly isolated from hospitalized patients, but it is important to distinguish between infection and simple colonization. This organism thrives in fluid-rich environments, making it particularly prone to colonizing body organs containing fluids. In hospitalized patients, Acinetobacter is frequently found in peritoneal fluid, cerebrospinal fluid (CSF), saliva, respiratory secretions, and the urinary tract. While its presence in these sites may indicate infection, it can also represent colonization, especially in critically ill or immunocompromised patients. Therefore, careful assessment is needed to determine if Acinetobacter is causing an active infection or merely colonizing these fluids. Misidentifying colonization as infection can lead to unnecessary treatments, while overlooking an actual infection may result in missed opportunities for appropriate therapy. Timely and accurate identification of the organism’s role in the patient's condition is crucial for effective management. Additionally, the risk of Acinetobacter outbreaks in healthcare settings underscores the importance of stringent infection control measures.
The pathogenesis and virulence of Acinetobacter are influenced by several key mechanisms. Acinetobacter species produce lipopolysaccharide (LPS) or lipooligosaccharide (LOS) in their outer membranes, with modifications in the synthesis of these structures contributing to antibiotic resistance and enhanced resistance to desiccation. Additionally, the presence of capsules helps protect the bacteria from complement-mediated killing, aiding in immune evasion. Pili on the surface of Acinetobacter play a critical role in its twitching motility, biofilm formation, and adherence to environmental surfaces, facilitating its persistence in hospital settings. Furthermore, Acinetobacter secretes various proteins that enhance its antibiotic resistance through mechanisms such as efflux pumps and enzymatic degradation of antimicrobial agents, making infections difficult to treat. These factors collectively contribute to the bacterium’s ability to persist in clinical environments and cause chronic, hard-to-eradicate infections.
Acinetobacter is known for its multidrug resistance, with cephalosporins, macrolides, and penicillins showing little to no activity against it. The use of these antibiotics can actually predispose patients to Acinetobacter infections. In cases where an infection is suspected, particularly in patients with long-term catheters or pacemakers, removal of the affected device is crucial for effective treatment. External devices, such as infected lines, shunts, or drains, must be removed to achieve a cure. Additionally, if the patient has abscesses or necrotic tissue, thorough debridement is necessary. Over recent years, drug resistance has become a significant concern, particularly in the U.S. Effective treatments may include meropenem, sulbactam/durlobactam, colistin, polymyxin B, and amikacin, with alternatives like minocycline, rifampin, and tigecycline available. Monotherapy is typically preferred, as combination therapy has not proven to be more effective. The duration of therapy generally ranges from 7 to 10 days, depending on the severity of the patient’s illness.
"Acinetobacter Infections- Pipeline Insight, 2025" report by DelveInsight outlays comprehensive insights of present scenario and growth prospects across the indication. A detailed picture of the Acinetobacter Infections pipeline landscape is provided which includes the disease overview and Acinetobacter Infections treatment guidelines. The assessment part of the report embraces, in depth Acinetobacter Infections commercial assessment and clinical assessment of the pipeline products under development. In the report, detailed description of the drug is given which includes mechanism of action of the drug, clinical studies, NDA approvals (if any), and product development activities comprising the technology, Acinetobacter Infections collaborations, licensing, mergers and acquisition, funding, designations and other product related details.
Report Highlights
- The companies and academics are working to assess challenges and seek opportunities that could influence Acinetobacter Infections R&D. The therapies under development are focused on novel approaches to treat/improve Acinetobacter Infections.
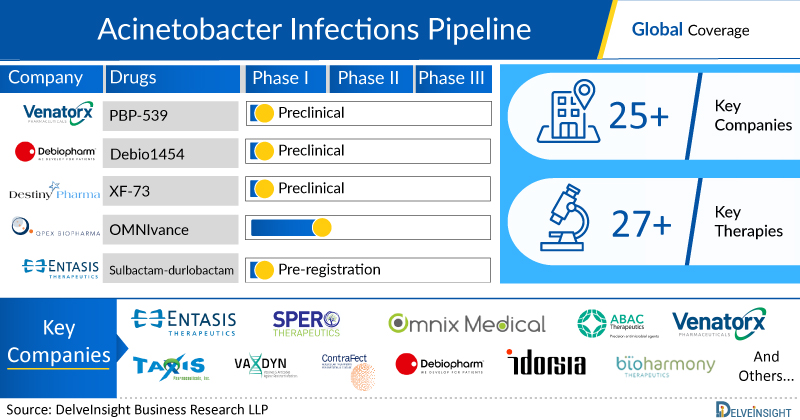
Acinetobacter Infections Emerging Drugs Chapters
This segment of the Acinetobacter Infections report encloses its detailed analysis of various drugs in different stages of clinical development, including Phase III, II, I, Preclinical and Discovery. It also helps to understand clinical trial details, expressive pharmacological action, agreements and collaborations, and the latest news and press releases.
Acinetobacter Infections Emerging Drugs
- OMN6: Omnix Medical Ltd
OMN6 is a novel, first-in-class antimicrobial peptide (AMP) based on insect host defense peptides. Its mechanism of action (MoA) is based on disruption of bacterial cell membranes and is therefore effective regardless of bacterial genotype or resistance phenotype, and unlike conventional bacteriostatic antibiotics, it is fast acting and bactericidal. OMN6 has been optimized from the original AMP by Omnix Medical´s proprietary technology to exhibit not only remarkable efficacy, potency, and safety, but also high stability while maintaining bioactivity. This novel peptide can be considered a new class of antimicrobial drugs. OMN6, the Company’s lead compound, is intended for the treatment of life-threatening infections caused by Gram-negative bacteria such as Acinetobacter baumannii. The US Food and Drug Administration (FDA) has also granted fast-track designation to OMN6. Currently, the drug is in Phase II stage of its development for the treatment of Acinetobacter Infections.
- BV100: Bioversys
BV100 has a novel mode of action addressing carbapenem resistant Acinetobacter baumannii (CRAB) lung and blood stream infections. CRAB is one of three highest priority pathogens on the WHO and CDC list and responsible for high mortality rates in intensive care units. BioVersys has developed and patented a new formulation of an already approved, safe drug that was previously unknown to be active on CRAB. Currently, the drug is in Phase II stage of its development for the treatment of Acinetobacter Infections.
- MRX-8: MicuRx
MRX-8 is a next-generation polymyxin antibiotic developed by MicuRx to target multidrug-resistant Gram-negative bacteria, including carbapenem-resistant Acinetobacter baumannii, Pseudomonas aeruginosa, Klebsiella pneumoniae, and other resistant pathogens. It was designed using a ""soft drug design"" approach, which integrates factors for metabolism and detoxification to enhance the drug's safety and therapeutic index. MRX-8 features a fatty acyl tail attached via an ester bond, enabling deesterification to a less toxic metabolite. Supported by the National Major Scientific and Technological Special Project for ""Significant New Drugs Development"" during the 13th five-year period, MRX-8 has shown promising results in clinical trials, with its US IND approved in 2020, Phase I trials completed in 2022, and Chinese Phase I trials completed in 2024. Currently, the drug is in Phase I stage of its development for the treatment of Acinetobacter Infections.
Further product details are provided in the report……..
Acinetobacter Infections: Therapeutic Assessment
This segment of the report provides insights about the different Acinetobacter Infections drugs segregated based on following parameters that define the scope of the report, such as:
Major Players in Acinetobacter Infections
There are approx. 8+ key companies which are developing the therapies for Acinetobacter Infections. The companies which have their Acinetobacter Infections drug candidates in the most advanced stage, i.e. Phase II include, Omnix Medical Ltd.
Phases
DelveInsight’s report covers around 10+ products under different phases of clinical development like
- Late stage products (Phase III)
- Mid-stage products (Phase II)
- Early-stage product (Phase I) along with the details of
- Pre-clinical and Discovery stage candidates
- Discontinued & Inactive candidates
Route of Administration
Acinetobacter Infections pipeline report provides the therapeutic assessment of the pipeline drugs by the Route of Administration. Products have been categorized under various ROAs such as
- Oral
- Intravenous
- Subcutaneous
- Parenteral
- Topical
Molecule Type
Products have been categorized under various Molecule types such as
- Recombinant fusion proteins
- Small molecule
- Monoclonal antibody
- Peptide
- Polymer
- Gene therapy
Product Type
Drugs have been categorized under various product types like Mono, Combination and Mono/Combination.
Acinetobacter Infections: Pipeline Development Activities
The report provides insights into different therapeutic candidates in Phase III, II, I, preclinical and discovery stage. It also analyses Acinetobacter Infections therapeutic drugs key players involved in developing key drugs.
Pipeline Development Activities
The report covers the detailed information of collaborations, acquisition and merger, licensing along with a thorough therapeutic assessment of emerging Acinetobacter Infections drugs.
Acinetobacter Infections Report Insights
- Acinetobacter Infections Pipeline Analysis
- Therapeutic Assessment
- Unmet Needs
- Impact of Drugs
Acinetobacter Infections Report Assessment
- Pipeline Product Profiles
- Therapeutic Assessment
- Pipeline Assessment
- Inactive drugs assessment
- Unmet Needs
Key Questions
Current Treatment Scenario and Emerging Therapies:
- How many companies are developing Acinetobacter Infections drugs?
- How many Acinetobacter Infections drugs are developed by each company?
- How many emerging drugs are in mid-stage, and late-stage of development for the treatment of Acinetobacter Infections?
- What are the key collaborations (Industry–Industry, Industry–Academia), Mergers and acquisitions, licensing activities related to the Acinetobacter Infections therapeutics?
- What are the recent trends, drug types and novel technologies developed to overcome the limitation of existing therapies?
- What are the clinical studies going on for Acinetobacter Infections and their status?
- What are the key designations that have been granted to the emerging drugs?

