Chronic Inducible Urticaria Market
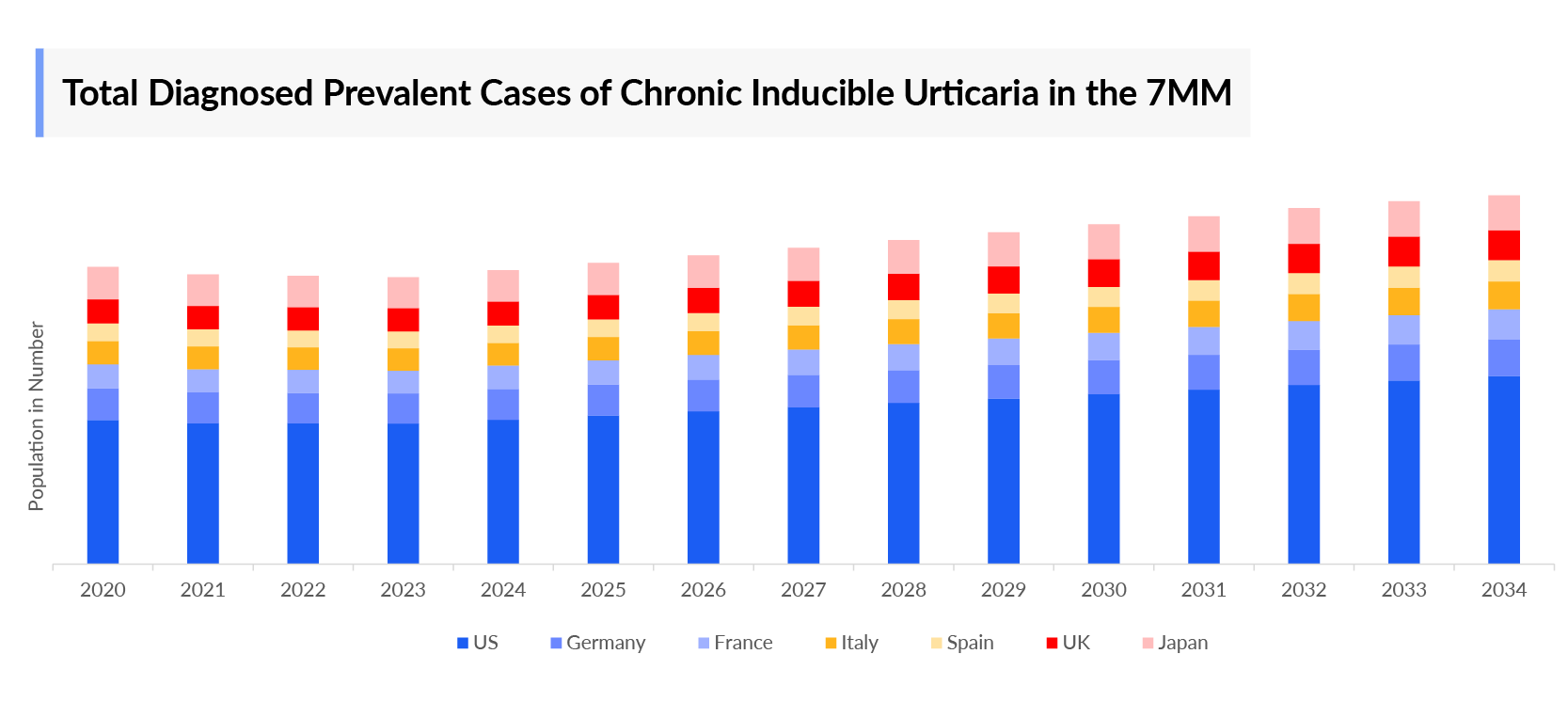
- According to DelveInsight’s estimates, in 2023, there were approximately 670 thousand total diagnosed prevalent cases of chronic inducible urticaria in the 7MM. Of these, the United States accounted for approximately 47% of the cases, while EU4 and the UK and Japan represented approximately 45% and 8% of the cases, respectively. These cases are expected to rise driven by factors such as the aging population, chronic diseases, medications and treatments, and improved lifestyle factors.
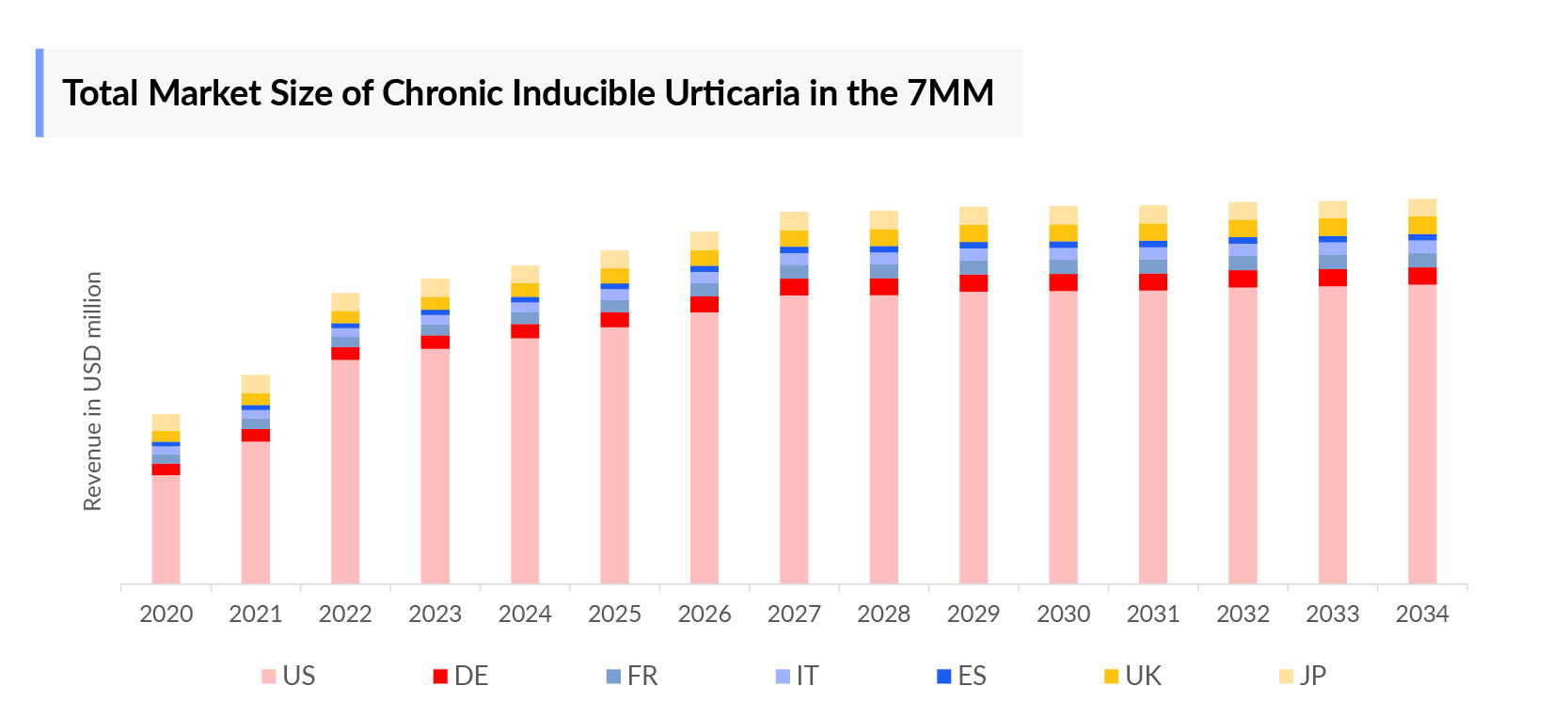
- The Chronic Inducible Urticaria Market is set for steady growth, with a robust compound annual growth rate (CAGR) anticipated from 2024 to 2034. This expansion in the 7MM is driven by the introduction of innovative therapies such as Briquilimab, Remibrutinib, CDX-0159, and others.
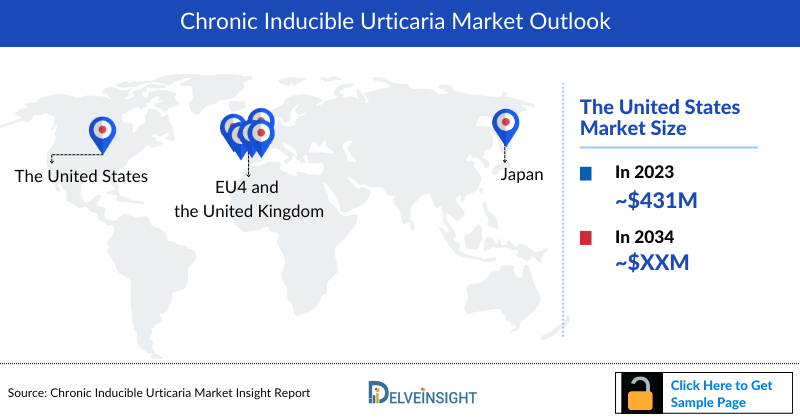
- According to DelveInsight’s analysis, the Chronic Inducible Urticaria Market in the US was valued at approximately USD 981 million in 2023. Over the forecast period from 2024 to 2034, this market is projected to grow at a compound annual growth rate (CAGR) of 12%.
- The Chronic Inducible Urticaria Therapeutics Market is marked by a significant unmet need due to the absence of FDA-approved therapies specifically targeting this condition. This gap underscores the potential for emerging drugs to fill a crucial therapeutic void, offering targeted, effective treatment options for CIndU patients who currently rely on off-label drugs and symptom management.
- Given the high unmet needs, key Chronic Inducible Urticaria Companies such as Novartis Pharmaceuticals, Jasper Therapeutics, Inc., and Celldex Therapeutics among others, who are actively advancing therapeutic and preventive solutions to address the growing prevalence and management of Chronic Inducible Urticaria.
Request for unlocking the sample page of the "Chronic Inducible Urticaria Treatment Market"
DelveInsight’s “Chronic Inducible Urticaria Market Insights, Epidemiology, and Market Forecast – 2034” report delivers an in-depth understanding of chronic inducible urticaria, historical and forecasted epidemiology, as well as the chronic inducible urticaria market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The Chronic Inducible Urticaria Treatment Market Report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM chronic inducible urticaria market size from 2020 to 2034. The report also covers Chronic Inducible Urticaria Treatment Market practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s potential.
| Study Period | 2020–2034 |
| Forecast Period | 2024–2034 |
| Geographies Covered | US, EU4 (Germany, France, Italy, and Spain) the UK, and Japan |
| Chronic Inducible Urticaria Epidemiology |
|
| Chronic Inducible Urticaria Market |
|
| Chronic Inducible Urticaria Market Analysis |
|
| Chronic Inducible Urticaria Companies |
|
| Future opportunity | A significant future opportunity for pharmaceutical companies lies in developing targeted biologics that inhibit specific pathways involved in mast cell activation and histamine release. Given the variability of triggers in chronic inducible urticaria, there is a need for treatments that can offer broader efficacy across different subtypes, such as cold, heat, or pressure-induced urticaria. Companies could focus on next-generation monoclonal antibodies or small molecule inhibitors that provide long-lasting relief and reduce the frequency of symptom flare-ups, addressing the unmet need for more effective and personalized treatment options in this challenging condition. |
Chronic Inducible Urticaria Treatment Market: Understanding and Treatment Algorithm
Chronic Inducible Urticaria (CIndU) represents a distinct subgroup of chronic urticaria, characterized by the recurrent emergence of wheals and/or angioedema in response to identifiable stimuli. These triggers are categorized into physical factors, such as symptomatic dermographism, cold and heat urticaria, delayed pressure urticaria, solar urticaria, and vibratory urticaria, and nonphysical factors, including cholinergic, contact, and aquagenic urticaria. The pathophysiology of CIndU is complex, often involving abnormal mast cell activation in response to these specific stimuli, leading to a cascade of inflammatory reactions. Understanding the precise triggers and underlying mechanisms is crucial for effective management and the development of targeted therapeutic strategies, highlighting the need for further research and innovation in this field.
Chronic Inducible Urticaria Diagnosis
The diagnosis of chronic inducible urticaria follows a structured approach, beginning with the exclusion of differential diagnoses through a comprehensive medical history and targeted diagnostic tests. Confirmation is achieved via provocation tests tailored to the suspected CIndU forms, with consideration given to the potential for multiple concurrent forms. These tests should be conducted with symptomatic therapies, such as antihistamines, discontinued for an optimal period to avoid skewed results. Provocation tests, along with trigger threshold determination, are crucial both before treatment initiation and during follow-up to assess disease progression. Disease control in CIndU is evaluated using the Urticaria Control Test (UCT), though the absence of disease-specific tools for assessing disease burden highlights a gap in current diagnostic methodologies.
Further details related to country-based variations are provided in the report…
Chronic Inducible Urticaria Treatment
Management of chronic inducible urticaria primarily involves trigger threshold testing and the avoidance of identified stimuli. In cases where avoidance is impractical, a stepwise symptomatic treatment approach is initiated, commencing with non-sedating H1-antihistamines, which may be increased up to four-fold for off-label use, as patients with CIndU often experience substantial benefits from higher dosing. However, when patients exhibit insufficient response to first and second-line therapies, third-line options, such as omalizumab, are recommended. Omalizumab, a recombinant humanized anti-IgE antibody, has gained approval as an add-on therapy for H1–antihistamine–refractory chronic spontaneous urticaria (CSU), suggesting potential efficacy for CIndU patients as well. Additionally, emerging therapies in the pipeline, including Briquilimab, remibrutinib, and CDX-0159, represent promising candidates for advancing treatment options and addressing the unmet needs in CIndU management, potentially enhancing patient outcomes and quality of life.
Chronic Inducible Urticaria Epidemiology
- As the market is derived using a patient-based model, the chronic inducible urticaria epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by Diagnosed Prevalent cases of CU, Diagnosed Prevalent cases of CIndU, Gender-specific Diagnosed Prevalent Cases of Chronic Inducible Urticaria, and Type-specific Diagnosed Prevalent Cases of Chronic Inducible Urticaria in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
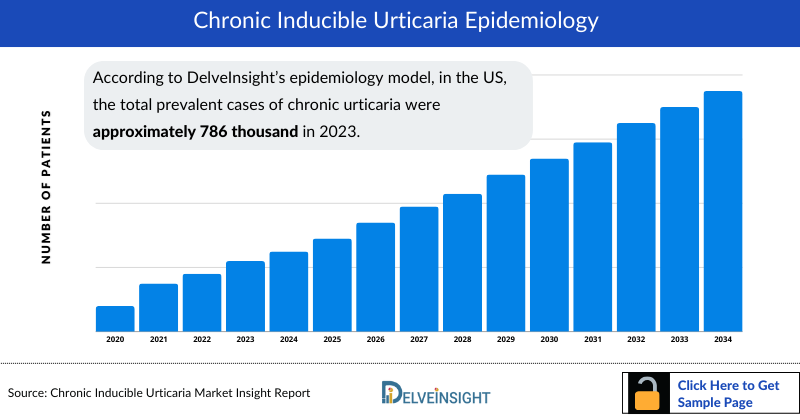
- According to DelveInsight’s epidemiology model, in the US, the total prevalent cases of chronic urticaria were approximately 786 thousand in 2023. This number is anticipated to rise during the forecast period (2024-2034), driven by increased awareness and screening, along with advancements in testing.
- In 2023, the US reported 314 thousand cases of diagnosed prevalent chronic inducible urticaria.
- In 2023, among the EU4 countries and the UK, Germany had the highest number of diagnosed prevalent cases of chronic inducible urticaria, with approximately 73 thousand cases. In contrast, Spain reported the lowest number, with around 47 thousand diagnosed cases.
- In 2023, chronic inducible urticaria in Germany shows a higher prevalence in females than males, likely due to hormonal differences and immune response variations that predispose women to urticaria.
- According to DelveInsight’s epidemiology model, Type-specific instances of chronic inducible urticaria in Japan are categorized into Dermographism, Cold urticaria, Cholinergic urticaria, and others where the highest cases of chronic inducible urticaria were attributed to dermographism with approximately 68 thousand in 2023.
Unlock comprehensive insights! Click Here to Purchase the Full Epidemiology Report @ Chronic Inducible Urticaria Prevalence
Chronic Inducible Urticaria Drug Chapters
Chronic Inducible Urticaria Emerging Drugs
- LOU064 (remibrutinib): Novartis
LOU064 (remibrutinib), an innovative immunology treatment under development by Novartis, is currently in Phase III clinical trials for CIndU. This potent Bruton's tyrosine kinase (BTK) inhibitor targets the underlying immune mechanisms responsible for CIndU by modulating B-cell receptor signaling and inhibiting mast cell activation. Remibrutinib mechanism of action offers a promising therapeutic approach for patients who are unresponsive to existing treatments, with the potential to provide more effective symptom control and improved quality of life for individuals suffering from this challenging condition.
- Barzolvolimab (CDX-0159): Celldex Therapeutics
Barzolvolimab (CDX-0159) is a humanized monoclonal antibody developed by Celldex Therapeutics that targets the receptor tyrosine kinase KIT with high specificity, potently inhibiting its activity. KIT is crucial in regulating mast cells, which mediate inflammatory responses like hypersensitivity and allergic reactions. In chronic urticaria, including Chronic Inducible Urticaria (CIndU), mast cell activation drives disease onset and progression. By inhibiting KIT signaling, Barzolvolimab aims to mitigate these inflammatory responses. Currently, in Phase III trials, it is being investigated for CIndU, Chronic Spontaneous Urticaria (CSU), prurigo nodularis (PN), and eosinophilic esophagitis (EOE), with potential future applications in atopic dermatitis (AD).
- EP262: Escient Pharmaceuticals
EP262, under development by Escient Pharmaceuticals is an innovative MRGPRX2 antagonist currently in Phase Ib/II trials targeting the treatment of CIndU. EP262 is a potent, highly selective once-daily small molecule antagonist of MRGPRX2, a receptor expressed on mast cells that is activated by numerous ligands, including many peptides released from sensory neurons as well as other cell types. In response to MRGPRX2 activation, mast cells release histamine, tryptase, chymase, chemokines, and cytokines, which can cause itchy hives, angioedema, type 2 inflammation (through engagement of the adaptive immune system) and chronic pruritus and pain. Escient’s preclinical data demonstrates that, by blocking the activation of MRGPRX2, EP262 has the potential to effectively treat a broad range of mast cell-mediated diseases, with an initial focus on chronic urticaria.
| Drug | MoA | RoA | Company | Logo | Phase |
| LOU064 (remibrutinib) | BTK inhibitor | Oral | Novartis |
| III |
| Barzolvolimab | KIT inhibitor | Intravenous | Celldex Therapeutics |
| III |
| EP262 | MRGPRX2 antagonist | Oral | Escient Pharmaceuticals, Inc |
| Ib/II |
| XX | XX | XX | XXX | XX | X |
Note: Further emerging therapies and their detailed assessment will be provided in the final report.
Chronic Inducible Urticaria Drugs Market Insights
The treatment of chronic inducible urticaria involves a strategic, multi-class approach to address the varying severity and persistence of the condition. Initial management typically begins with non-sedating H1-antihistamines, which act as the first-line therapy to mitigate histamine-mediated symptoms. For patients unresponsive to standard doses, the treatment regimen may be escalated, often involving off-label up-dosing of these antihistamines. In cases where antihistamines prove insufficient, third-line therapies are introduced, such as omalizumab, a recombinant humanized anti-IgE antibody, targeting the underlying immunological mechanisms of urticaria. Emerging classes of treatments in development include Bruton's tyrosine kinase (BTK) inhibitors like remibrutinib, which interfere with intracellular signaling pathways critical to immune cell activation, and KIT antagonist monoclonal antibodies such as Barzolvolimab (CDX-0159), which specifically inhibit mast cell activity—a key driver in CIndU pathogenesis. This evolving therapeutic landscape underscores the need for targeted interventions to improve patient outcomes.
Chronic Inducible Urticaria Market Outlook
The therapeutic management of chronic inducible urticaria aims to achieve complete symptom control through trigger avoidance, desensitization, and the inhibition of mast cell mediators. Currently, no approved therapies exist specifically for CIndU, necessitating a reliance on symptomatic management strategies. The treatment paradigm predominantly includes the stepwise application of second-generation antihistamines, utilized in standard doses as first-line therapy and escalated up to four-fold for second-line treatment. Additionally, off-label use of omalizumab, an anti-IgE monoclonal antibody initially approved for chronic spontaneous urticaria (CSU), and cyclosporine are considered for refractory cases.
Other medications employed include doxepin hydrochloride, histamine-2 blockers, and antileukotrienes, though their individual market shares remain largely indeterminate due to variability in clinical practice and the prevalence of generics. Moreover, the lack of clinical efficacy data for cyclosporine and alternative agents further complicates market assessments. Consequently, the current market forecast focuses primarily on the revenues generated from symptomatic therapies and the off-label use of Omalizumab (Xolair), whether administered alone or in combination. Emerging therapeutic agents, including remibrutinib, a BTK inhibitor, and Barzolvolimab (CDX-0159), a KIT antagonist, represent promising advancements in the treatment landscape for CIndU, potentially enhancing management outcomes and addressing unmet clinical needs.
Chronic Inducible Urticaria Drugs Uptake
This section focuses on the uptake rate of potential Chronic Inducible Urticaria drugs expected to be launched in the market during 2020–2034. For example, LOU064 (remibrutinib) is expected to enter the US market in 20XX and is projected to have a XX uptake during the forecast period.
Chronic Inducible Urticaria Pipeline Development Activities
The Chronic Inducible Urticaria therapeutics market report provides insights into different therapeutic candidates in Phase III, Phase II, and Phase I. It also analyzes key Chronic Inducible Urticaria Companies involved in developing targeted therapeutics.
Pipeline development activities
The Chronic Inducible Urticaria therapeutics market report covers information on collaborations, acquisitions and mergers, licensing, and patent details for emerging therapies for chronic inducible urticaria.
Take Your Research to the Next Level! Click Here to Get Access to the Full Pipeline Report @ Chronic Inducible Urticaria Treatment Drugs
KOL Views
To keep up with current market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on chronic inducible urticaria evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, including Medical/scientific writers, Medical Professionals, Professors, Directors, and Others.
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Centers like the Department of Dermatology and Allergy, Comprehensive Allergy Center, Hannover Medical School, Hannover, Germany and Instituto de Investigación Sanitaria de Navarra Pamplona (IDISNA), Spain, RETIC de Asma, Reacciones Adversas y Alérgicas, Madrid (ARADyAL), Spain, Madrid, Spain among others, were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or chronic inducible urticaria market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Chronic Inducible Urticaria Drugs Market: Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyzes multiple emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
To analyze the effectiveness of these therapies, have calculated their attributed analysis by giving them scores based on their ability to improve atrial and ventricular dimension/function and ability to regulate heart rate. Further, the therapies’ safety is evaluated wherein the adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials, which directly affects the safety of the molecule in the upcoming trials. It sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Chronic Inducible Urticaria Therapeutics Market Access and Reimbursement
The high cost of therapies for the treatment is a major factor restraining the growth of the global drug market. Because of the high cost, the economic burden is increasing, leading the patient to escape from proper treatment. The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Further details will be provided in the report.
The Chronic Inducible Urticaria Therapeutics Market Report provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenarios, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Chronic Inducible Urticaria Treatment Market Report Scope
- The Chronic Inducible Urticaria treatment market report covers a segment of key events, an executive summary, and a descriptive overview explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines have been provided.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the chronic inducible urticaria market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The Chronic Inducible Urticaria treatment market report provides an edge while developing business strategies by understanding trends through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM chronic inducible urticaria drugs market.
Chronic Inducible Urticaria Treatment Market Report Insights
- Patient-based Chronic Inducible Urticaria Market Forecasting
- Therapeutic Approaches
- Chronic Inducible Urticaria Pipeline Analysis
- Chronic Inducible Urticaria Market Size and Trends
- Existing and Future Chronic Inducible Urticaria Drugs Market Opportunity
Chronic Inducible Urticaria Treatment Market Report Key Strengths
- 11 years Chronic Inducible Urticaria Market Forecast
- The 7MM Coverage
- Chronic Inducible Urticaria Epidemiology Segmentation
- Key Cross Competition
- Attribute analysis
- Chronic Inducible Urticaria Drugs Uptake
- Key Chronic Inducible Urticaria Market Forecast Assumptions
Chronic Inducible Urticaria Treatment Market Report assessment
- Current Chronic Inducible Urticaria Treatment Market Practices
- Chronic Inducible Urticaria Unmet Needs
- Chronic Inducible Urticaria Pipeline Product Profiles
- Chronic Inducible Urticaria Drugs Market Attractiveness
- Qualitative Analysis (SWOT and Attribute Analysis)
Key Questions
Chronic Inducible Urticaria Treatment Market Insights
- What was the total Chronic Inducible Urticaria treatment market, the Chronic Inducible Urticaria market size by therapies, and market share (%) distribution in 2020, and what would it look like by 2034? What are the contributing factors for this growth?
- How will LOU064 (remibrutinib) affect the treatment paradigm of chronic inducible urticaria?
- Which drug is going to be the largest contributor by 2034?
- How would future opportunities affect the market dynamics and subsequent analysis of the associated trends?
Chronic Inducible Urticaria Epidemiology Insights
- What are the disease risks, burdens, and Chronic Inducible Urticaria Unmet Needs? What will be the growth opportunities across the 7MM with respect to the patient population pertaining to chronic inducible urticaria?
- What is the historical and forecasted chronic inducible urticaria patient pool in the United States, EU4 (Germany, France, Italy, and Spain) the United Kingdom, and Japan?
- Out of the countries mentioned above, which country would have the highest diagnosed prevalent chronic inducible urticaria population during the forecast period (2024–2034)?
- What factors are contributing to the growth of chronic inducible urticaria cases?
Current Chronic Inducible Urticaria Treatment Market Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options for the treatment of chronic inducible urticaria? What are the current clinical and treatment guidelines for treating chronic inducible urticaria?
- How many companies are developing therapies for the treatment of chronic inducible urticaria?
- How many emerging therapies are in the mid-stage and late stage of development for treating chronic inducible urticaria?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- What is the cost burden of current treatment on the patient?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the accessibility issues of approved therapy in the US?
- What is the 7MM historical and forecasted market of chronic inducible urticaria?
Reasons to Buy
- The Chronic Inducible Urticaria treatment market report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the chronic inducible urticaria Drugs Market.
- Insights on patient burden/disease Chronic Inducible Urticaria Prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- The distribution of historical and current patient share is based on real-world prescription data in the US, EU4 (Germany, France, Italy, and Spain) the United Kingdom, and Japan.
- Identifying upcoming solid players in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of Access and Reimbursement policies for chronic inducible urticaria, barriers to accessibility of approved therapy, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.
Stay updated with us for Recent Articles @ Latest DelveInsight Blogs

-02.png)