Cystinosis Market
- The Cystinosis Market Dynamics are poised to change with increased competition among pharmaceutical companies, potentially leading to improved accessibility, affordability, and innovation in treatment options. Additionally, advancements in gene therapy and precision medicine may reshape treatment paradigms.
- Cystinosis Epidemiology is expected to grow due to improved diagnostics, leading to increased awareness and earlier detection. Additionally, advancements in treatments prolonging patient survival may contribute to a larger patient population over time.
- Standard Cystinosis Treatment includes cysteamine bitartrate therapy, depleting cystine via immediate or delayed-release forms. PROCYSBI and CYSTADROPS are approved for managing cystinosis by preventing cystine accumulation.
- Nephropathic or infantile cystinosis is the most common type, typically diagnosed in infancy. This form of cystinosis results from mutations in the CTNS gene, leading to impaired cystine transport in lysosomes. Without proper treatment, nephropathic cystinosis can progress to renal failure, highlighting the importance of early diagnosis and intervention.
- Cystinosis Emerging Therapies, including gene therapy and novel targeted treatments, hold promise for Cystinosis patients. These therapies aim to address the underlying genetic cause of the disease, offering potential curative approaches or more effective long-term management strategies.
Request for Unlocking the Sample Page of the "Cystinosis Treatment Market"
DelveInsight’s comprehensive report titled “Cystinosis Market Insights, Epidemiology, and Market Forecast – 2034” offers a detailed analysis of Cystinosis. The report presents historical and projected epidemiological data covering Total Prevalent cases of Cystinosis, Diagnosed Prevalent cases of Cystinosis and Type-specific Diagnosed Prevalent cases of Cystinosis. In addition to epidemiology, the market report encompasses various aspects related to the patient population. These aspects include the diagnosis process, prescription patterns, physician perspectives, market accessibility, treatment options, and prospective developments in the market across seven major markets: the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan, spanning from 2020 to 2034.
The Cystinosis Therapeutics Market Report analyzes the existing treatment practices and unmet medical requirements in Cystinosis. It evaluates the market potential and identifies potential business prospects for enhancing therapies or interventions. This valuable information enables stakeholders to make well-informed decisions regarding product development and strategic planning for the market.
| Study Period | 2020–2034 |
| Forecast Period | 2024–2034 |
| Geographies Covered |
|
| Cystinosis Epidemiology |
|
| Cystinosis Drugs Market |
|
| Cystinosis Market Analysis |
|
| Cystinosis Companies |
|
| Challenges | It include limited awareness leading to delayed diagnosis, high treatment costs burdening healthcare systems, and lifelong therapy requirements impacting patient adherence. Additionally, the rarity of the disease poses challenges in conducting clinical trials and developing new therapies. |
Cystinosis Treatment Market: Overview
Cystinosis is a rare genetic disorder characterized by the accumulation of the amino acid cystine within cells throughout the body. It primarily affects the kidneys and eyes, although other organs and tissues may also be involved. There are three main types of cystinosis: nephropathic or infantile, intermediate, and non-nephropathic or ocular. Cystinosis is caused by mutations in the CTNS gene, which encodes a protein responsible for transporting cystine out of cells. Without functional CTNS protein, cystine accumulates within lysosomes, forming crystals that damage cells and tissues. While cystinosis is inherited in an autosomal recessive pattern, some cases may occur sporadically without a family history. Common symptoms of cystinosis include poor growth, dehydration, electrolyte imbalances, and frequent urination. Early diagnosis and management are crucial in preventing complications and improving quality of life for individuals with cystinosis.
Nephropathic cystinosis, the most severe form, typically presents in infancy, leading to kidney dysfunction and failure if left untreated. Intermediate cystinosis usually manifests later in childhood or adolescence and may involve milder kidney impairment compared to the nephropathic form. Non-nephropathic cystinosis primarily affects the eyes, causing photophobia, corneal crystals, and progressive vision loss.
Cystinosis Diagnosis and Treatment Algorithm
Diagnosis of cystinosis involves clinical evaluation, including assessment of symptoms such as poor growth, dehydration, and electrolyte imbalances, along with family history and genetic testing. Initial laboratory tests may reveal elevated levels of cystine in white blood cells or urine, providing supportive evidence. Ophthalmologic examination can detect corneal cystine crystals, particularly in non-nephropathic cystinosis cases. Confirmation of diagnosis is achieved through genetic testing to identify mutations in the CTNS gene, which encodes the cystinosin protein responsible for cystine transport. Early diagnosis enables prompt initiation of treatment to mitigate complications and improve outcomes.
The current standard treatment for cystinosis involves cystine-depleting therapy using cysteamine bitartrate, administered orally as immediate-release or delayed-release formulations. Cysteamine works by breaking down cystine into cysteine and cysteine-cysteamine mixed disulfide, reducing cystine accumulation within cells and preventing crystal formation. PROCYSBI (delayed-release cysteamine bitartrate) and CYSTAGON (immediate-release cysteamine bitartrate) are approved medications for cystinosis management.
An emerging therapy, CRISPR/Cas9 gene editing, holds promise for correcting CTNS gene mutations, addressing the underlying cause of cystinosis. This innovative approach aims to restore cystinosin function, potentially offering a curative treatment option. Further research and clinical trials are underway to assess the safety and efficacy of CRISPR/Cas9 gene editing in cystinosis patients, with encouraging preliminary results.
Cystinosis Epidemiology
The epidemiology section on the Cystinosis treatment market report offers information on the patient populations, including historical and projected trends for each of the seven major markets. Examining key opinion leader views from physicians or clinical experts can assist in identifying the reasons behind historical and projected trends. The diagnosed patient pool, their trends, and the underlying assumptions are all included in this section of the report.
This section also presents the data with relevant tables and graphs, offering a clear and concise view of the prevalence of Cystinosis. Additionally, the report discloses the assumptions made during the analysis, ensuring data interpretation and presentation transparency. This epidemiological data is valuable for understanding the disease burden and its impact on the patient population across various regions.
Key Findings
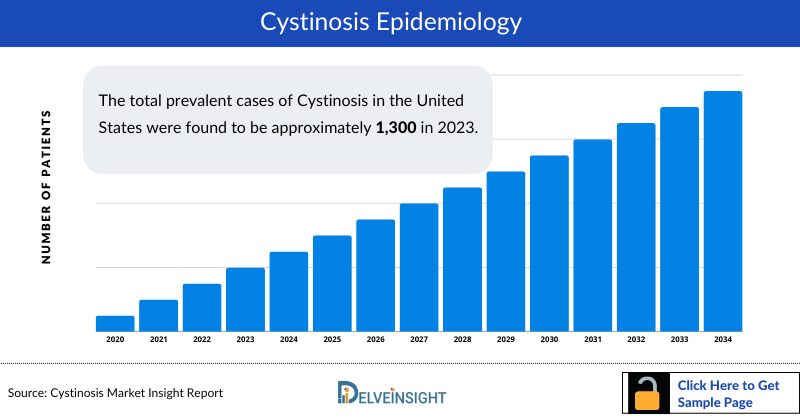
- The total Cystinosis Prevalent Cases in the United States were found to be approximately 1,300 in 2023.
- Total Cystinosis Prevalent Population in the EU4 and the UK was observed to be approximately 1600 in 2023. Whereas, in Japan the prevalent cases were estimated to be around 55 in 2023.
- United States had the highest Cystinosis Diagnosed Prevalent Cases with approximately 680 cases in 2023. In EU4 and the UK, the UK had the highest number of cases, followed by Germany, France and Spain. On the other hand, Italy accounted for the least number of cases in 2023.
- There were approximately 1250 cases of Infantile nephropathic cystinosis, 65 cases of Juvenile nephropathic cystinosis, and 45 cases of Adult-onset (Ocular, or non-nephropathic cystinosis) in the 7MM.
Unlock comprehensive insights! Click Here to Purchase the Full Epidemiology Report @ Cystinosis Prevalence
Cystinosis Market Outlook
The primary treatment goal for cystinosis is to reduce cystine accumulation within cells, preventing tissue damage and delaying disease progression. Achieving and maintaining low cystine levels is essential in managing symptoms, preserving kidney function, and improving overall quality of life for patients with cystinosis.
Current standard therapy for cystinosis employs cystine-depleting medications like cysteamine bitartrate. Two approved drugs for this purpose include PROCYSBI (delayed-release cysteamine bitartrate) and CYSTAGON (immediate-release cysteamine bitartrate). They function by breaking down cystine, lessening its harmful effects on tissues and organs. Meanwhile, CYSTADROPS (cysteamine hydrochloride) by Recordati S.p.A. offers targeted treatment for corneal cystine crystals, addressing ocular manifestations directly through localized therapy via eye drops.
An emerging therapy for cystinosis is CRISPR/Cas9 gene editing, currently in development by various companies. This innovative approach aims to correct mutations in the CTNS gene, restoring the function of the cystinosin protein responsible for cystine transport. By addressing the underlying genetic cause of cystinosis, CRISPR/Cas9 gene editing offers the potential for a curative treatment option. This therapy involves delivering CRISPR components into target cells to precisely edit the CTNS gene, enabling normal cystine transport and reducing cystine accumulation. While still in the early stages of development, CRISPR/Cas9 gene editing holds promise for transforming the treatment landscape of cystinosis, offering hope for improved outcomes and potentially a lifelong cure.
With ongoing research and continued dedication, the future holds hope for even more effective treatments and, ultimately, a cure for this challenging condition. "According to DelveInsight, the Cystinosis Therapeutics Market in the 7MM is expected to change significantly during the study period 2020–2034"
Cystinosis Drug Chapters
Marketed Cystinosis Drugs
- PROCYSBI (cysteamine bitartrate) : Horizon Pharmaceutical/Chiesi Farmaceutici
PROCYSBI (cysteamine bitartrate), developed by Horizon Pharmaceutical/Chiesi Farmaceutici, is a delayed-release formulation approved by the FDA in 2013 for treating cystinosis. Administered orally, it acts as a cystine-depleting therapy, breaking down cystine into less harmful components, thereby reducing cystine accumulation within cells. Procysbi offers convenient dosing with twice-daily administration and aims to mitigate complications associated with cystinosis, improving patient outcomes.
- CYSTADROPS (cysteamine hydrochloride) : Recordati S.p.A
CYSTADROPS (cysteamine hydrochloride), developed by Recordati S.p.A., is an ophthalmic solution approved in 2018 for treating corneal cystine crystals in patients with Cystinosis. Administered as eye drops, its mechanism of action involves breaking down cystine crystals in the cornea, reducing their accumulation and alleviating symptoms of photophobia and vision impairment. CYSTADROPS offer targeted treatment directly to the affected tissues, providing localized therapy for ocular manifestations of Cystinosis.
Emerging Cystinosis Drugs
The Cystinosis therapeutics market is expected to experience gradual changes, mainly due to the limited availability of emerging therapies in this area. Key market players, including Nacuity Pharmaceuticals, Inc. and others, have demonstrated a keen interest in this rare condition and are actively pursuing the development of potential treatments.
- NPI-001 : Nacuity Pharmaceuticals, Inc.
NPI-001, or N-acetylcysteine amide, is an investigational drug developed by Nacuity Pharmaceuticals, Inc. for potential use in Cystinosis and other conditions. N-acetylcysteine amide is a modified form of N-acetylcysteine (NAC), a well-known antioxidant and precursor to glutathione synthesis. While NAC has shown promise in various medical applications, N-acetylcysteine amide offers improved bioavailability and potentially enhanced therapeutic effects due to its modified structure. It is hypothesized that NPI-001 may help reduce oxidative stress and cystine accumulation in cells, thereby mitigating tissue damage and disease progression in Cystinosis. Clinical trials are likely underway to evaluate the safety and efficacy of NPI-001, with the goal of providing a novel treatment option for Cystinosis patients.
| Drug | MoA | RoA | Company | Phase |
| NPI-001 | Cysteamine Inhibitor | Oral | Nacuity Pharmaceuticals, Inc. | I/II |
| XXX | XXX | XXX | XXX | XX |
Note: Detailed emerging therapies assessment will be provided in the final report.
Cystinosis Market Segmentation
DelveInsight’s ‘Cystinosis Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a detailed outlook of the current and future Cystinosis market, segmented within countries, and by therapies. Further, the market of each region is then segmented by each therapy to provide a detailed view of the current and future market share of all therapies.
Cystinosis Treatment Market Size by Countries
The Cystinosis treatment market size is assessed separately for various countries, including the United States, EU4 (Germany, France, Italy, and Spain), the UK, and Japan. In 2023, the United States held a significant share of the overall 7MM (Seven Major Markets) Cystinosis market, primarily attributed to the country's higher prevalence of the condition and the elevated cost of the available treatments. This dominance is projected to persist, especially with the potential early introduction of new products.
Cystinosis Treatment Market Size by Therapies
Cystinosis Market Size by Therapies is categorized into current and emerging markets for the study period 2020–2034. One of the emerging drugs anticipated to launch during the forecast period is NPI-001 under the developmental pipeline of Nacuity Pharmaceuticals, Inc.
Note: Detailed market segment assessment will be provided in the final report.
Cystinosis Drugs Uptake
This section focuses on the sales uptake of potential Cystinosis drugs that have recently been launched or are anticipated to be launched in the Cystinosis market between 2020 and 2034. It estimates the market penetration of Cystinosis drugs for a given country, examining their impact within and across classes and segments. It also touches upon the financial and regulatory decisions contributing to the probability of success (PoS) of the drugs in the Cystinosis market.
Note: Detailed assessment of drug uptake will be provided in the full report on Cystinosis
Cystinosis Therapeutics Market Access and Reimbursement
DelveInsight’s ‘Cystinosis Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a descriptive overview of the market access and reimbursement scenario of Cystinosis. This section includes a detailed analysis of the country-wise healthcare system for each therapy, enlightening the market access, reimbursement policies, and health technology assessments.
KOL Views
To keep up with current Cystinosis market trends and fill gaps in secondary findings, we interview KOLs and SMEs’ working in the Cystinosis domain. Their opinion helps understand and validate current and emerging therapies and treatment patterns or Cystinosis market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the Cystinosis unmet needs.
Cystinosis : KOL Insights
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. These KOLs were from organizations, institutes, and hospitals, such as the Medical Biochemical Genetic Section, National Institutes of Health, Bethesda, MD USA, National Human Genome Research Institute, NIH, USA, Department of Pediatric Subspecialties, Division of Nephrology, Bambino Gesù Children’s Hospital, Rome, Italy, Department of Clinical Sciences and Community Health, University of Milan, Italy, Department of Pediatrics, Kobe University Graduate School of Medicine, Kobe, Japan, Department of Pathology, Yokohama City University Medical Center, Yokohama, Kanagawa, Japan, Manchester Royal Eye Hospital and Manchester Academic and Health Sciences Centre, Manchester, UK, Quinze-Vingts National Ophthalmology Hospital, Paris, France, Department of Medicine, University Hospital Freiburg, Hugstetter, Germany, among others.
Emphasizing early diagnosis to mitigate complications, highlighting the importance of cystine-depleting therapy in managing symptoms and preserving renal function, also giving attention to continued research into emerging treatments like CRISPR/Cas9 gene editing to address the underlying genetic cause, aiming for more personalized and curative approaches in Cystinosis management.
Note: Detailed assessment of KOL Views will be provided in the full report on Cystinosis
Competitive Intelligence Analysis
We conduct a Competitive and Cystinosis Drugs Market Intelligence analysis of the Cystinosis Market, utilizing various Competitive Intelligence tools such as SWOT analysis, Conjoint Analysis, and Market entry strategies. The inclusion of these analyses is contingent upon data availability, ensuring a comprehensive and well-informed assessment of the market landscape and competitive dynamics.
The emerging Cystinosis therapies are analyzed based on various attributes such as safety and efficacy in randomized clinical trials, order of entry and other market dynamics, and the unmet need they fulfill in the Cystinosis drugs market.
Note: Detailed assessment of SWOT analysis and Conjoint analysis will be provided in the full report on Cystinosis
Cystinosis Pipeline Development Activities
The Cystinosis therapeutics market report offers an analysis of therapeutic candidates in Phase I and and examines Cystinosis Companies involved in developing targeted therapeutics for Cystinosis. It provides valuable insights into the advancements and progress of potential treatments in clinical development for this condition.
Pipeline Development Activities
The Cystinosis therapeutics market report covers information on collaborations, acquisition and merger, licensing, patent details, and other information for emerging Cystinosis therapies.
Take Your Research to the Next Level! Click Here to Get Access to the Full Pipeline Report @ Cystinosis Treatment Drugs
Cystinosis Therapeutics Market Report Insights
- Patient-based Cystinosis Market Forecasting
- Therapeutic Approaches
- Cystinosis Pipeline Drugs Analysis
- Cystinosis Market Size and Trends
- Cystinosis Drugs Market Opportunities
- Impact of Upcoming Therapies
Cystinosis Therapeutics Market Report Key Strengths
- 11 Years Cystinosis Market Forecast
- The 7MM Coverage
- Cystinosis Epidemiology Segmentation
- Key Cross Competition
- Highly Analyzed Cystinosis Drugs Market
- Cystinosis Drugs Uptake
Cystinosis Treatment Market Report Assessment
- Current Cystinosis Treatment Market Practices
- Cystinosis Unmet Needs
- Cystinosis Pipeline Drugs Analysis Profiles
- Cystinosis Drugs Market Attractiveness
Key Questions
- How common is Cystinosis?
- What are the key findings of Cystinosis epidemiology across the 7MM, and which country will have the highest number of patients during the study period (2020–2034)?
- What are the currently available treatments for Cystinosis?
- What is the disease risk, burden, and Cystinosis Unmet Needs?
- At what CAGR is the Cystinosis drugs market and its epidemiology expected to grow in the 7MM during the forecast period (2024–2034)?
- How would the unmet needs impact the Cystinosis market dynamics and subsequently influence the analysis of the related trends?
- What would be the forecasted patient pool of Cystinosis in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), the UK, and Japan?
- Among EU4 and the UK, which country will have the highest number of patients during the forecast period (2024–2034)?
- How many companies are currently developing therapies for the treatment of Cystinosis?
Stay Updated with us for Recent Articles