Endometrial Cancer Market
Key Highlights
- The Endometrial Cancer Market Size is anticipated to grow with a significant CAGR during the study period (2019-2032).
- According to the National Cancer Institute, approximately 3 in 100 women will be diagnosed with uterine cancer at some point in their lives. Endometrial cancer comprises about 4% of all cancers in women globally.
- Endometrial cancer epidemiology is segmented as Total Endometrial Cancer Incident Cases, Endometrial Cancer Stage-specific Incident Cases, Endometrial Cancer Age-specific Cases and Total Endometrial Cancer Treated Cases by line of therapies] in the Endometrial Cancer market report.
- In February 2025, the OncoSignature multiplex immunofluorescence assay received FDA breakthrough device designation for identifying endometrial cancer patients who may benefit from ACR-368 (prexasertib) treatment.
- In February 2025, Acrivon Therapeutics announced that the FDA granted Breakthrough Device designation for its ACR-368 OncoSignature assay. This multiplex immunofluorescence assay is designed to identify endometrial cancer patients who may benefit from ACR-368 treatment.
Request for unlock CAGR of Endometrial Cancer Market
DelveInsight's "Endometrial Cancer Market Insights, Epidemiology, and Market Forecast — 2032" report delivers an in-depth understanding of the Endometrial Cancer, historical and forecasted epidemiology as well as the Endometrial Cancer therapeutics market trends in the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom and Japan.
The Endometrial Cancer market report provides current treatment practices, emerging drugs, Endometrial Cancer market share of the individual therapies, current and forecasted Endometrial Cancer market Size from 2019 to 2032 segmented by seven major markets. The Report also covers current Endometrial Cancer treatment practice/algorithm, and unmet medical needs to curate best of the opportunities and assesses the underlying potential of the market.
| Study Period | 2019 to 2032 |
| Forecast Period | 2023-2032 |
| Geographies Covered |
|
| Endometrial Cancer Market |
|
| Endometrial Cancer Market Size | |
| Endometrial Cancer Companies | GlaxoSmithKline Pharmaceuticals, Merck & Co, AstraZeneca, Karyopharm Therapeutics, Evergreen Therapeutics, Incyte Corporation, and others. |
| Endometrial Cancer Epidemiology Segmentation |
|
Endometrial Cancer Treatment Market
The DelveInsight’s Endometrial Cancer market report gives a thorough understanding of Endometrial Cancer symptoms, risks, causes, pathophysiology, diagnosis, and treatment. Endometrial cancer develops when cells in the endometrium (the inner lining of the uterus) begin to grow out of control. Cancerous cells can arise in almost any portion of the body and spread to other parts. Endometrial cancer is sometimes called uterine cancer. It is often detected at an early stage because it frequently produces abnormal vaginal bleeding.
If endometrial cancer is discovered early, removing the uterus surgically often cures endometrial cancer. The most common type of endometrial cancer (type 1) grows slowly. It most often is found only inside the uterus. Type 2 is less common. It grows more rapidly and tends to spread to other parts of the body.
The most common symptom of endometrial cancer is abnormal vaginal bleeding. This include changes in the length or heaviness of menstrual periods, vaginal bleeding or spotting between menstrual periods, vaginal bleeding after menopause. Other potential symptoms of endometrial cancer include: watery or blood-tinged vaginal discharge, pain in the lower abdomen or pelvis, pain during sex
Endometrial Cancer Diagnosis
Tests and procedures used to diagnose endometrial cancer include: examining the pelvis, using sound waves to create a picture of the uterus, using a scope to examine the endometrium, removing a sample of tissue for testing and performing surgery to remove tissue for testing. When a person is diagnosed with endometrial cancer, the stage of the cancer affects what treatment options are available and the long-term outlook. Endometrial cancer is easier to treat in the early stages of the condition.
Endometrial Cancer Treatment
Treatment options and recommendations for endometrial cancer depend on several factors, including the type and stage of cancer, possible side effects, overall health, age, and personal preferences. This includes whether or how treatment will affect one’s fertility. Uterine cancer is treated by one or a combination of treatments, including surgery, radiation therapy, and systemic treatments using medications. Combinations of these cancer treatments are often recommended, but they depend on the stage and characteristics of the cancer.
Surgery, radiation therapy, chemotherapy, hormone therapy, targeted drug therapy, immunotherapy and supportive (palliative) care are some of the treatment options available for treating Endometrial Cancer. Total hysterectomy with bilateral salpingo-oophorectomy is the standard treatment for endometrial cancer. Treatment options include radiation and chemotherapy. Nonsurgical treatments are available for low- to medium-risk endometrial hyperplasia. The stage of the disease and histology are used to determine survival, with most patients in stages I and II having a good prognosis. Obesity, diabetes, and hypertension are all risk factors for endometrial cancer, therefore controlling them could help prevent it.
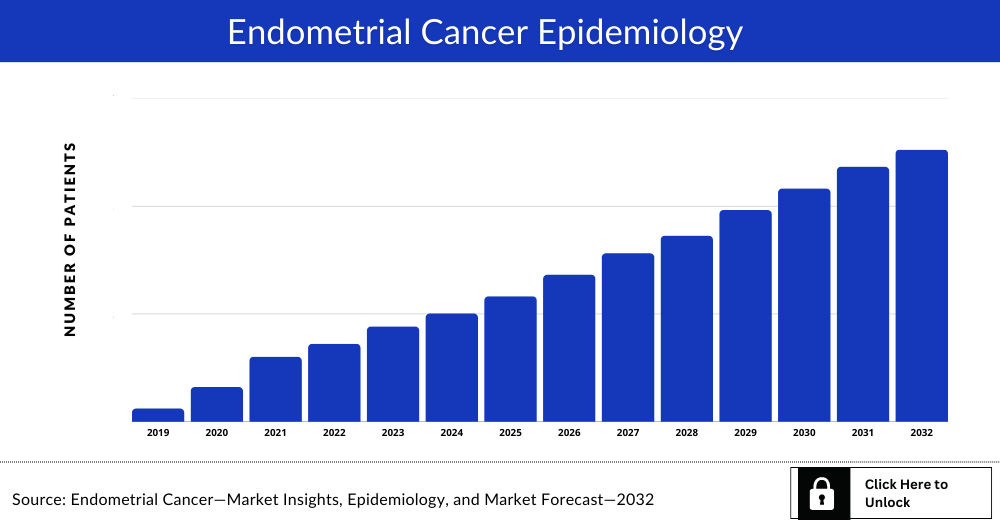
Endometrial Cancer Epidemiology
The Endometrial Cancer epidemiology section provides insights about historical and current Endometrial Cancer patient pool and forecasted trends for individual seven major countries. It helps to recognize the causes of current and forecasted trends by exploring numerous studies and views of key opinion leaders. This part of the report also provides the diagnosed patient pool and their trends along with assumptions undertaken. Endometrial cancer incidence by age increases significantly after menopause, with the highest cases reported in women over 50 years old.
Key Findings
- The disease epidemiology covered in the report provides historical as well as forecasted Endometrial cancer epidemiology scenario in the 7MM covering the United States,EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan from 2019 to 2032.
- According to the National Cancer Institute, approximately 3 in 100 women will be diagnosed with uterine cancer at some point in their lives. Endometrial cancer comprises about 4% of all cancers in women globally.
- The disease epidemiology covered in the report provides historical as well as forecasted Endometrial cancer epidemiology [segmented as Total Incident Cases of Endometrial Cancer, Stage-specific Incident Cases of Endometrial Cancer, Age-specific Cases of Endometrial Cancer and Total Treated Cases of Endometrial Cancer by line of therapies] in the 7MM covering the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan from 2019 to 2032.
Country Wise- Endometrial Cancer Epidemiology
This section provides a glimpse of the Endometrial Cancer epidemiology in the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan.
Discover key insights on Endometrial Cancer prevalence! Stay updated on prevalence, trends, and future projections for better awareness and care.
Endometrial Cancer Recent Developments
- In February 2025, the OncoSignature multiplex immunofluorescence assay received FDA breakthrough device designation for identifying endometrial cancer patients who may benefit from ACR-368 (prexasertib) treatment.
- In February 2025, Acrivon Therapeutics announced that the FDA granted Breakthrough Device designation for its ACR-368 OncoSignature assay. This multiplex immunofluorescence assay is designed to identify endometrial cancer patients who may benefit from ACR-368 treatment.
Endometrial Cancer Drug Chapters
The drug chapter segment of the Endometrial Cancer report encloses the detailed analysis of Endometrial Cancer marketed drugs and late stage (Phase-III and Phase-II) pipeline drugs. It also helps to understand the Endometrial Cancer clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest news and press releases. The endometrial cancer pipeline drugs market is expanding with innovative therapies, offering hope for improved treatment outcomes and patient survival rates.
Endometrial Cancer Marketed Drugs
JEMPERLI (dostarlimab-gxly): GlaxoSmithKline Pharmaceuticals
JEMPERLI is a programmed death receptor-1 (PD-1)–blocking antibody and its binding of the PD-1 ligands, PD-L1 and PD-L2, to the PD-1 receptor found on T cells inhibits T-cell proliferation and cytokine production. Up regulation of PD-1 ligands occurs in some tumors, and signaling through this pathway can contribute to inhibition of active T-cell immune surveillance of tumors. Dostarlimab is a humanized monoclonal antibody of the IgG4 isotype that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, releasing PD-1 pathway-mediated inhibition of the immune response, including the anti-tumor immune response.
KEYTRUDA (pembrolizumab): Merck & Co
KEYTRUDA (pembrolizumab) binding of the PD-1 ligands, PD-L1 and PD-L2, to the PD-1 receptor found on T cells, inhibits T cell proliferation and cytokine production. Upregulation of PD-1 ligands occurs in some tumors and signaling through this pathway can contribute to inhibition of active T-cell immune surveillance of tumors. It is a monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, releasing PD-1 pathway-mediated inhibition of the immune response, including the anti-tumor immune response. In syngeneic mouse tumor models, blocking PD-1 activity resulted in decreased tumor growth.
Note: Detailed Current therapies assessment will be provided in the full report of Endometrial Cancer...
Endometrial Cancer Emerging Drugs
AstraZeneca is conducting Phase III of Lynparza (Olaparib) + Imfinzi DuO-E is an inhibitor of poly (ADP-ribose) polymerase (PARP) enzymes, including PARP1, PARP2, and PARP3. PARP enzymes are involved in normal cellular homeostasis, such as DNA transcription, cell cycle regulation, and DNA repair. It has been shown to inhibit growth of select tumor cell lines in vitro and decrease tumor growth in mouse xenograft models of human cancer both as monotherapy or following platinum-based chemotherapy. Increased cytotoxicity and anti-tumor activity following treatment with olaparib were noted in cell lines and mouse tumor models with deficiencies in BRCA. In vitro studies have shown that olaparib-induced cytotoxicity may involve inhibition of PARP enzymatic activity and increased formation of PARP-DNA complex, resulting in disruption of cellular homeostasis and cell death.
Karyopharm Therapeutics is conducting a Phase III randomized, prospective, multicenter, double-blind, placebo-controlled study to obtain evidence of efficacy for maintenance selinexor in participants with advanced or recurrent endometrial cancer. Participants with primary stage IV or recurrent disease who are in partial or complete response after having completed a single line of at least 12 weeks of taxane-platinum combo therapy will be randomized in a 2:1 manner to maintenance therapy with 80 milligram (mg) with selinexor once weekly (QW) or placebo until progression. The study is expected to be completed by March 2023.
Evergreen Therapeutics is conducting Phase III of EG-007 is an injectable drug used to treat endometrial cancer. Endometrial cancer is an epithelial malignant tumor occurring in the endometrium and is one of the three malignant tumors of the female reproductive system. Although immunotherapy drugs account for more than 60% of clinical trials in endometrial cancer, the low response rate of immune checkpoint drugs is an urgent problem to be solved in clinical practice. Animal experiments showed that EG-007 could enhance the therapeutic effect of anti-PD-1 /L1 antibody, which was related to enhancing the infiltration of CD8+ T cells, up-regulating IFN-γ signaling pathway, and improving the immunosuppressive microenvironment in tumor tissues. The company’s goal is to develop EG-007 in combination with immunotherapy as a first-line treatment for advanced endometrial cancer.
Discover the future of Endometrial Cancer treatments! Explore emerging therapies and key players in the Endometrial Cancer Pipeline Insight, 2025!
Endometrial Cancer Market Outlook
Treatment options and recommendations for endometrial cancer depend on several factors, including the type and stage of cancer, possible side effects, overall health, age, and personal preferences. This includes whether or how treatment will affect one’s fertility. Uterine cancer is treated by one or a combination of treatments, including surgery, radiation therapy, and systemic treatments using medications. Combinations of these cancer treatments are often recommended, but they depend on the stage and characteristics of the cancer.
Significant developments are being made in endometrial cancer therapy over the past several decades. Many monotherapies and combinations are in trial. Companies across the globe are diligently working toward the development of novel Endometrial Cancer treatment therapies with a considerable amount of success over the years. AstraZeneca, Karyopharm Therapeutics, Evergreen Therapeutics, and Incyte Corporation are some of the key players developing drugs for the treatment of Endometrial Cancer.
In March 2021, Eisai Inc. launched an initiative called Spot Her to end the silence around endometrial cancer and inspire women to listen, advocate, and put their health and the health of other women first. The initiative was conducted in partnership with SHARE Cancer Support (SHARE), Facing Our Risk of Cancer Empowered (FORCE), and Black Health Matters. Moreover, in November 2021, the UNC Lineberger Comprehensive Cancer Center launched the Endometrial Cancer Center of Excellence to advance the scientific understanding of the causes, prevention, and Endometrial Cancer clinical trial treatment and aid researchers in discovering the underlying factors that contribute to why black women in North Carolina are twice as likely to die from endometrial cancer. Such developments spread awareness about endometrial cancer, thereby boosting the demand for the endometrial cancer treatment market.
The dynamics of Endometrial Cancer is anticipated to change in the coming years owing to the improvement in the rise in the number of healthcare spending across the world.
According to DelveInsight, the Endometrial Cancer market in 7MM is expected to witness a major change in the study period 2019-2032.
Analyst Commentary
- The pipeline is very robust, many potential Endometrial Cancer therapies are being investigated for the treatment and better outcome, and it is safe to predict that the treatment space will experience a significant impact on the market during the forecast period.
- The expected introduction of emerging therapies with improved efficacy, more awareness initiatives programs, and further improvement in the diagnosis rate, are likely to boost the growth of the Endometrial Cancer market in the 7MM. Aside from that, the market size of Endometrial Cancer may flourish due to increased research and development, label-expansion of approved therapies into other epilepsy in this field.
- The market growth of Endometrial Cancer may be offset by failures and/or discontinuation of the emerging therapies, unaffordable pricing, market access and reimbursement issues, and a scarcity of healthcare specialists.
Endometrial Cancer Drugs Uptake
This section focuses on the rate of uptake of the potential drugs recently launched in the Endometrial Cancer market or expected to get launched in the market during the study period 2019-2032. The analysis covers Endometrial Cancer market uptake by drugs; patient uptake by therapies; and sales of each drug.
This helps in understanding the Endometrial Cancer drugs with the most rapid uptake, reasons behind the maximal use of new drugs, and allows the comparison of the drugs based on market share and size which again will be useful in investigating factors important in market uptake and in making financial and regulatory decisions.
Note: Detailed emerging therapies assessment will be provided in the full report of Endometrial Cancer...
Endometrial Cancer Pipeline Development Activities
The report provides insights into different Endometrial Cancer clinical trials within Phase II and Phase III stage. It also analyses Endometrial Cancer key players involved in developing targeted therapeutics.
Endometrial Cancer Clinical Trial Development Activities
The report covers the detailed information of collaborations, acquisition and merger, licensing, patent details and other information for Endometrial Cancer emerging therapies.
Reimbursement Scenario in Endometrial Cancer
Approaching reimbursement proactively can have a positive impact both during the late stages of product development and well after product launch. In a report, we consider reimbursement to identify economically attractive indications and market opportunities. When working with finite resources, the ability to select the markets with the fewest reimbursement barriers can be a critical business and price strategy.
KOL- Views
To keep up with current market trends, we take KOLs and SMEs ' opinions working in the Endometrial Cancer domain through primary research to fill the data gaps and validate our secondary research. Their opinion helps to understand and validate current and emerging therapies treatment patterns or Endometrial Cancer market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the Endometrial Cancer unmet needs.
Competitive Intelligence Analysis
We perform Competitive and Market Intelligence analysis of the Endometrial Cancer Market by using various Competitive Intelligence tools that include - SWOT analysis, PESTLE analysis, Porter's five forces, BCG Matrix, Market entry strategies etc. The inclusion of the analysis entirely depends upon the data availability.
Scope of the Endometrial Cancer Market Report
- The report covers the descriptive overview of Endometrial Cancer, explaining its causes, signs and symptoms, pathophysiology, diagnosis, and currently available therapies
- Comprehensive insight has been provided into the Endometrial Cancer epidemiology and treatment in the 7MM
- Additionally, an all-inclusive account of both the current and emerging therapies for Endometrial Cancer are provided, along with the assessment of new therapies, which will have an impact on the current treatment landscape
- A detailed review of the Endometrial Cancer market; historical and forecasted is included in the report, covering drug outreach in the 7MM
- Detailed Patient Based Market Forecasting determines the trends shaping and driving the Global Endometrial Cancer market
Endometrial Cancer Market Report Highlights
- In the coming years, the Endometrial Cancer market is set to change due to the rising awareness of the disease, and incremental healthcare spending across the world; which would expand the size of the market to enable the drug manufacturers to penetrate more into the market
- The companies and academics are working to assess challenges and seek opportunities that could influence Endometrial Cancer R&D. The therapies under development are focused on novel approaches to treat/improve the disease condition
- Major players are involved in developing therapies for Endometrial Cancer. The launch of emerging therapies will significantly impact the Endometrial Cancer market
- A better understanding of disease pathogenesis will also contribute to the development of novel therapeutics for Endometrial Cancer
- Our in-depth analysis of the pipeline assets across different stages of development (Phase III and Phase II), different emerging trends, and comparative analysis of pipeline products with detailed Endometrial Cancer clinical trial profiles, key cross-competition, launch date along with product development activities will support the clients in the decision-making process regarding their therapeutic portfolio by identifying the overall scenario of the research and development activities
Endometrial Cancer Report Insights
- Endometrial Cancer Patient Based Market Forecasting
- Endometrial Cancer Therapeutic Approaches
- Endometrial Cancer Pipeline Analysis
- Endometrial Cancer Market Size and Trends
- Endometrial Cancer Market Opportunities
- Impact of Upcoming Endometrial Cancer Therapies
- Endometrial Cancer Drugs Market
Endometrial Cancer Report Key Strengths
- 11 Years Forecast
- 7MM Coverage
- Endometrial Cancer Epidemiology Segmentation
- Key Cross Competition
- Highly Analyzed Market
- Endometrial Cancer Drugs Uptake
Endometrial Cancer Report Assessment
- Current Endometrial Cancer Treatment Practices
- Endometrial Cancer Unmet Needs
- Endometrial Cancer Pipeline Product Profiles
- Endometrial Cancer Market Attractiveness
- Endometrial Cancer Market Drivers
- Endometrial Cancer Market Barriers
Key Questions Answered In The Endometrial Cancer Market Report:
Endometrial Cancer Market Insights:
- What was the Endometrial Cancer drug class share (%) distribution in 2019 and how it would look like in 2032?
- What would be the Endometrial Cancer total market size as well as market size by therapies across the 7MM during the forecast period (2019-2032)?
- What are the key findings of the market across 7MM and which country will have the largest Endometrial Cancer market size during the forecast period (2019-2032)?
- At what CAGR, the Endometrial Cancer market is expected to grow by 7MM during the forecast period (2019-2032)?
- What would be the Endometrial Cancer market outlook across the 7MM during the forecast period (2019-2032)?
- What would be the Endometrial Cancer market growth till 2032, and what will be the resultant market Size in the year 2032?
- How would the unmet needs affect the market dynamics and subsequent analysis of the associated trends?
Endometrial Cancer Epidemiology Insights:
- What are the disease risk, burden, and regional/ethnic differences of Endometrial Cancer?
- What are the key factors driving the epidemiology trend for seven major markets covering the United States, EU4 (Germany, Spain, Italy, and France) and the UK, and Japan?
- What is the historical Endometrial Cancer patient population pool in seven major markets covering the United States, EU4 (Germany, Spain, Italy, and France) and the UK, and Japan?
- What would be the forecasted patient pool of Endometrial Cancer in seven major markets covering the United States, EU4 (Germany, Spain, Italy, and France) and the UK, and Japan?
- Where will be the growth opportunities in the 7MM concerning the patient population about Endometrial Cancer?
- Out of all 7MM countries, which country would have the highest incident population of Endometrial Cancer during the forecast period (2019-2032)?
- At what CAGR the patient population is expected to grow by 7MM during the forecast period (2019-2032)?
Current Endometrial Cancer Treatment Scenario, Marketed Drugs, and Emerging Therapies:
- What are the current options for the Endometrial Cancer treatment in addition to the approved therapies?
- What are the current treatment guidelines for the treatment of Endometrial Cancer in the US, Europe, and Japan?
- What are the Endometrial Cancer marketed drugs and their respective MOA, regulatory milestones, product development activities, advantages, disadvantages, safety, efficacy, etc.?
- How many Endometrial Cancer companies are developing therapies for the treatment and better treatment?
- How many therapies are in-development by each company for Endometrial Cancer treatment?
- How many are emerging therapies in mid-stage, and late stage of development for Endometrial Cancer treatment?
- What are the key collaborations (Industry - Industry, Industry-Academia), Mergers and acquisitions, licensing activities related to the Endometrial Cancer therapies?
- What are the recent novel therapies, targets, mechanisms of action, and technologies being developed to overcome the limitation of existing therapies?
- What are the clinical studies going on for Endometrial Cancer and its status?
- What are the current challenges faced in drug development?
- What are the key designations that have been granted for the emerging therapies for Endometrial Cancer?
- What are the global historical and forecasted markets of Endometrial Cancer?
Reasons to buy Endometrial Cancer Market Forecast Report
- The Patient Based Market Forecasting analysis will help in developing business strategies by understanding trends shaping and driving the Endometrial Cancer market
- Organize sales and marketing efforts by identifying the best opportunities for Endometrial Cancer in the US, Europe (Germany, Spain, Italy, and France) and the United Kingdom, and Japan
- Identification of strong upcoming players in the market will help in devising strategies that will help in getting ahead of competitors
- Organize sales and marketing efforts by identifying the best opportunities for the Endometrial Cancer market
- To understand the future market competition in the Endometrial Cancer market