external defibrillators market
External Defibrillators Market by Product Type (Non-Wearable [Semi-Automated and Automated] and Wearable), End-User (Hospitals, Ambulatory Surgical Centers, Home Care Settings, and Others), and Geography (North America, Europe, Asia-Pacific, and Rest of the World) are expected to grow at a steady CAGR forecast till 2030 owing to the rising burden of cardiac diseases, increasing awareness and screening programs, increase in product launches and approvals by key market players across the globe.
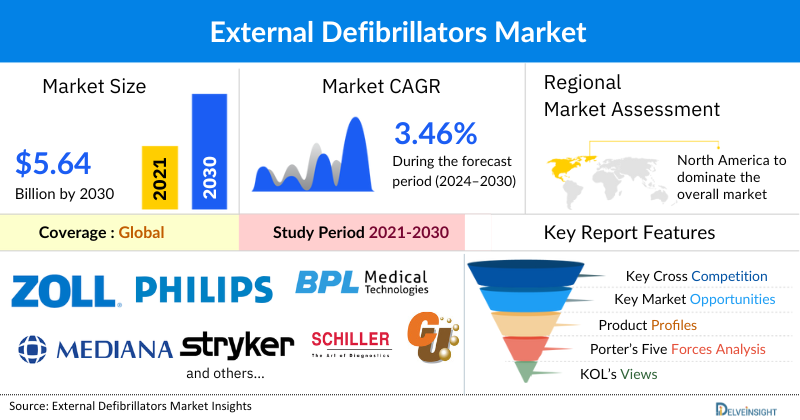
The external defibrillators market was valued at USD 4.60 billion in 2023, growing at a CAGR of 3.46% during the forecast period from 2024 to 2030 to reach USD 5.64 billion by 2030. The global incidence of cardiovascular diseases (CVDs) is rising due to lifestyle changes, smoking, lack of physical activities, excessive alcohol consumption, obesity, and other factors that are escalating the global market of external defibrillators. Cardiac diseases often lead to sudden cardiac arrest, where the immediate availability of a defibrillator can be crucial in saving lives. The growing prevalence of these diseases heightens the demand for external defibrillators, both in healthcare settings and public spaces. Additionally, public health campaigns and initiatives aimed at raising awareness about cardiovascular health encourage individuals to undergo regular check-ups and screenings. Increased public knowledge about the importance of early detection and management of heart diseases leads to higher utilization of external defibrillators. Furthermore, key market players continuously investing in research and development, increased partnerships and collaborations, product launches, and approvals ensure that the market remains dynamic and innovative, during the forecast period from 2024 to 2030.
External Defibrillators Market Dynamics:
According to recent data provided by the British Heart Foundation (2024), globally, approximately 620 million people, or about 1 in 13 individuals, live with heart and circulatory diseases. Additionally, as per the same source, the prevalence of heart and circulatory diseases was 100 million in Europe and 340 million in Asia and Australia in 2021.
Additionally, according to the World Heart Federation (2024), approximately 60 million population are affected by atrial fibrillation which is one of the most common types of irregular heartbeat, or arrhythmia. It can increase the risk of blood clots, heart failure, and stroke.
Additionally, as per the same source people with atrial fibrillation are 5X times more likely to suffer a stroke.
Furthermore, according to the recent data and stats provided by the Australian Institute of Health and Welfare (2024), in 2021, more than 500,000 population were affected by atrial fibrillation.
According to a recent data provided by British Heart Foundation (2024), globally, around 56 million women and 45 million men are stroke survivors. It is estimated that at least 13 million people worldwide live with congenital heart disease, with potentially millions more undiagnosed.
Thus, the increasing incidence of heart and circulatory diseases, atrial fibrillation, and stroke survivors creates a heightened demand for external defibrillators as these conditions often result in sudden cardiac arrest (SCA) and irregular cardiac rhythm, where immediate defibrillation can be lifesaving thus boosting the overall market of external defibrillators.
Additionally, product developmental activities across the globe play a pivotal role in accelerating the market growth of external defibrillators. For instance, in June 2021, Rapid Response Revival (RRR) earned CE mark certification in Europe for their automated external defibrillator (AED), CellAED.
Thus, the factors mentioned above are likely to boost the market of external defibrillators during the forecasted period.
However, the electric shock delivered by a defibrillator can cause burns or skin injuries at the electrode pad contact points, and stringent regulatory concerns for product approval may hinder the future market of external defibrillators.
External Defibrillators Segment Analysis:
External Defibrillators Market by Product Type (Non-Wearable [Semi-Automated and Automated] and Wearable), End-User (Hospitals, Ambulatory Surgical Centers, Home Care Settings, and Others), and Geography (North America, Europe, Asia-Pacific, and Rest of the World).
In the product type segment of the external defibrillators market, wearable is projected to hold a considerable market share in 2023. Wearable defibrillators, also known as Wearable Cardioverter-Defibrillators (WCDs), are playing a significant role in boosting the overall market for external defibrillators. WCDs are used for patients who are at high risk of sudden cardiac arrest. This includes patients with recent heart attacks, those with severe heart failure, or individuals with transient arrhythmias. The availability of wearable defibrillators enhances safety and confidence among patients at risk of sudden cardiac arrest. Knowing that they have continuous monitoring and the potential for immediate defibrillation can reduce anxiety and encourage patients to engage more actively with their healthcare providers.
Wearable defibrillators often come with advanced data collection and integration features that allow for better monitoring and management of cardiac health. This integration into healthcare systems can lead to improved protocols and practices that benefit all types of defibrillators. The data generated from WCDs can provide insights into the effectiveness of defibrillation strategies, leading to more informed decisions and increased investment in defibrillator technologies.
Additionally, the ongoing technological advancements in wearable defibrillators, product approvals, and launches across the globe have further escalated the market of the segment. For instance, in August 2021, Kestra™ Medical Technologies, Inc. announced that the company received premarket approval from the U.S. Food and Drug Administration (FDA) for the ASSURE® Wearable Cardioverter Defibrillator (WCD) system. Thus, these innovations attract healthcare providers looking to invest in state-of-the-art equipment to deliver high-quality care thereby boosting the market of wearable defibrillators across the globe.
Therefore, owing to the above-mentioned factors, the wearable defibrillators category is expected to generate considerable revenue thereby pushing the overall growth of the global external defibrillators market during the forecast period.
North America is expected to dominate the overall External Defibrillators Market:
North America is expected to account for the highest proportion of the external defibrillators market in 2023, out of all regions. This can be ascribed to the increasing prevalence of cardiac disease, increased government initiatives coupled with increased awareness programs for cardiac disease, and the presence of key market players engaged in mergers, acquisitions, product launches, and other market activities across the region are expected to escalate the market of external defibrillators.
According to the recent data provided by the Centre for Disease Control and Prevention (2024), in 2022, approximately 4.9% of adults have been diagnosed with coronary heart disease.
Additionally, as per the recent data provided by the Centre for Disease Control and Prevention (2024), approximately 12.1 million population in the United States will have atrial fibrillation by 2023.
Additionally, as per the same source each year, over 795,000 individuals in the United States experience a stroke. Of these, approximately 610,000 are first-time or new strokes. Nearly 185,000 strokes, accounting for almost 1 in 4, occur in people who have already had a stroke.
Thus, the increasing incidence of heart and circulatory diseases, atrial fibrillation, and stroke survivors creates a heightened demand for external defibrillators as these conditions often result in sudden cardiac arrest (SCA) and irregular cardiac rhythm, where immediate defibrillation can be lifesaving thus boosting the overall market of external defibrillators.
Additionally, the awareness programs in the United States aimed at addressing cardiac diseases focus on education, prevention, and early detection and also boost the market of external defibrillators across the region as public awareness campaigns educate individuals about the importance of regular screenings and preventive measures. For example, February is designated as American Heart Month, during which various organizations, including the American Heart Association (AHA) and the Centers for Disease Control and Prevention (CDC), promote awareness through campaigns, events, and educational materials. These efforts emphasize the importance of heart health, risk factors, and preventive measures.
In addition, various commercialization activities including collaborations, product launches, mergers, acquisitions, and others in the field of External Defibrillators are also projected to fuel the market in the region. For instance, in January 2023, Avive Solutions, Inc., received U.S. Food and Drug Administration (FDA) pre-market approval (PMA for its Avive AED™, a unique Automated External Defibrillator (AED).
Therefore, the above-mentioned factors are expected to bolster the growth of the external defibrillators market in North America during the forecast period.
External Defibrillators Market Key Players:
Some of the key market players operating in the external defibrillators market include ZOLL Medical Corporation, Koninklijke Philips N.V., BPL Medical Technologies, Mediana Co., Ltd, CU Medical System Inc., Stryker, Shenzhen Mindray Bio-Medical Electronics Co. Ltd, SCHILLER, Bexen Cardio, Nihon Kohden Corporation, Shenzhen Comen Medical Instruments Co., Ltd., Progetti S.r.l., Starker Medical SL, HEARTHERO, Kestra Medical Technologies, Inc., Trivitron Healthcare, Amiitalia, Shanghai Huifeng Medical Instrument Co., Ltd, Metrax GmbH, Meditech Equipment Co ., Ltd., and others.
Recent Developmental Activities in the External Defibrillators Market:
- In January 2024, Element Science received a CE mark in Europe and approval in the U.K. for its patch-based cardioverter defibrillator, designed to offer a more wearable option for individuals at risk of sudden cardiac arrest who may not qualify for or prefer not to have a long-term implant.
- In October 2023, Medtronic plc received FDA approval for its Aurora EV-ICD™ MRI SureScan™ Extravascular Implantable Cardioverter-Defibrillator and Epsila EV™ MRI SureScan™ defibrillation lead. These devices are designed to treat dangerously fast heart rhythms that can lead to sudden cardiac arrest (SCA).
Key Takeaways from the Market Report Study
- Market size analysis for current external defibrillators size (2023), and market forecast for 6 years (2024 to 2030)
- Top key product/technology developments, mergers, acquisitions, partnerships, and joint ventures happened over the last 3 years
- Key companies dominating the external defibrillators market.
- Various opportunities available for the other competitors in the external defibrillators market space.
- What are the top-performing segments in 2023? How these segments will perform in 2030?
- Which are the top-performing regions and countries in the current external defibrillators market scenario?
- Which are the regions and countries where companies should have concentrated on opportunities for external defibrillator market growth in the coming future?
Target Audience who can be benefited from this Market Report Study
- External defibrillator product providers
- Research organizations and consulting companies
- External defibrillators -related organizations, associations, forums, and other alliances
- Government and corporate offices
- Start-up companies, venture capitalists, and private equity firms
- Distributors and traders dealing in external defibrillators
- Various end-users who want to know more about the external defibrillators market and the latest technological developments in the external defibrillators market.