Factor D Inhibitor Market Forecast
- Factor D inhibitor market size is expected to increase at a steady growth rate, owing to anticipated commercial success of emerging and marketed therapies in multiple complement dysregulated disorders during our forecast period (2024-2034).
- Factor D, also referred to as C3 proactivator convertase, constitutes the activating enzyme of the C3 convertase of the alternative pathway. As such, it is an essential component of initiation and amplification of the pathway.
- The function of Factor D is to cleave its unique substrate, Factor B in Mg++ dependent complex with C3(H2O) or C3b, to generate the alternative pathway C3 convertases C3(H2O)Bb and C3bBb. Therefore, factor D is a key and rate-limiting component in the alternative pathway. It also participates in the amplification loop which contributes significantly to responses elicited by the complement classical and lectin pathways.
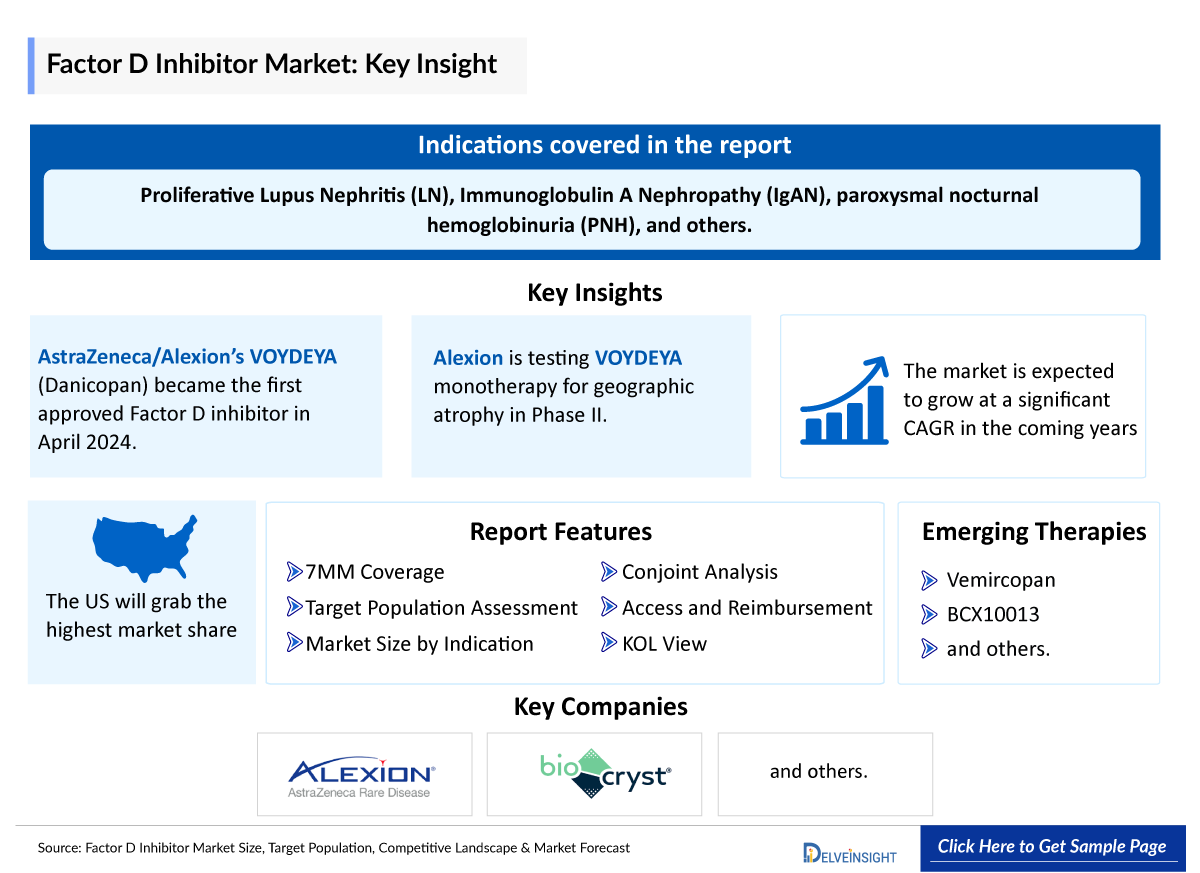
- In April 2024, AstraZeneca/Alexion’s VOYDEYA (Danicopan) became the first Factor D inhibitor to get approval as an add-on therapy to ULTOMIRIS (ravulizumab) or SOLIRIS (eculizumab) for treatment of extravascular haemolysis in adults with paroxysmal nocturnal hemoglobinuria (PNH).
- Alexion is also evaluating VOYDEYA as a potential monotherapy for geographic atrophy in a Phase II clinical trial.
- Currently, AstraZeneca/Alexion’s vemircopan (second generation Factor D) and BioCryst’s BCX10013 are under clinical development for complement mediated renal diseases and PNH, respectively.
DelveInsight’s “Factor D Inhibitor Market Size, Target Population, Competitive Landscape and Market Forecast - 2034” report delivers an in-depth understanding of Factor D Inhibitor, addressable patient pool, competitive landscape, and future market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the UK, and Japan.
The Factor D Inhibitor market report provides insights around existing treatment practices in patients with Factor D Inhibitor, approved (if any) and emerging Factor D Inhibitor, market share of individual therapies, patient pool eligible for treatment with Factor D Inhibitor, along with current and forecasted 7MM Factor D Inhibitor market size from 2020-2034 by therapies and by indication. The Factor D inhibitor market report also covers current unmet needs and challenges while incorporating new classes in treatment paradigm, variations in accessibility and acceptability of new Factor D Inhibitor in different geographies, along with insights on Factor D Inhibitor pricing reimbursements to curate the best opportunities and assess the Factor D inhibitor market’s potential.
Factor D Inhibitor Market: Understanding
Factor D Complement Inhibitors Overview
Factor D is produced by adipocytes and secreted into circulation. Once MASP-3 has cleaved off the propeptide and converted factor D into its mature form, factor D is ready to perform its essential function in both the initiation phase and the amplification phase of the alternative complement pathway. First, factor D cleavage of C3-bound factor B is responsible for generation of the C3(H2O)Bb convertase, which splits C3 into C3b and the pro-inflammatory signaling anaphylatoxin C3a. Second, factor D cleavage of C3b-bound factor B in the amplification loop is responsible for generation of the predominant C3 convertase C3bBb, which amplifies the signal and creates additional C3b and C3a molecules. The classical and lectin complement pathways converge at the amplification loop of the alternative pathway and as such their signals are also amplified by the action of factor D. Besides incorporation into C3 convertase complexes for signal amplification, C3b also functions to opsonize cells for phagocytosis and, via downstream reactions, initiates MAC formation and cell lysis.
Further details related to Factor D complement inhibitors overview are provided in the report
Factor D Complement Inhibitors Treatment
Factor D and the alternative complement pathway have been implicated in both healthy states and disease states. Under normal conditions, the pathway helps to protect against invading pathogens and maintain homeostasis and health of various tissues and organs. However, upon dysregulation, the alternative complement pathway can contribute to the pathogenesis of various diseases throughout the body. Although these diseases can affect specific organs or tissues, many of them are also characterized by systemic complications and widespread inflammation.
AstraZeneca/Alexion’s VOYDEYA (danicopan), administered orally, is the only marketed Factor D complement inhibitor in PNH. Alexion is also evaluating another Factor D inhibitor, vemircopan, in patients with proliferative lupus nephritis (LN) or immunoglobulin A nephropathy (IgAN).
Besides, Alexion, other companies such as BioCryst are using the PNH patient pool as a benchmark for assessing the effectiveness of their Factor D complement inhibitor. Currently, BioCryst is investigating BCX10013 in a proof-of-concept trial involving individuals with paroxysmal nocturnal hemoglobinuria (PNH) to validate its potential. Following this, the company intends to progress into a pivotal trials targeting patients with renal complement-mediated diseases, such as IgA nephropathy (IgAN).
Further details related to treatment overview are provided in the report
Factor D Complement Inhibitors Drug Chapters
The drug chapter segment of the Complement Inhibitors market reports encloses a detailed analysis of Complement Inhibitors’ marketed drugs and emerging drugs (Phase I and Phase II). It also helps understand the Factor D inhibitors' clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug and the latest news and press releases.
Marketed Factor D inhibitor Drugs
Currently, VOYDEYA (danicopan), administered orally, is the only marketed Factor D complement inhibitor in PNH.
VOYDEYA (danicopan) : AstraZeneca/Alexion
In April 2024, FDA approved VOYDEYA as add-on therapy to ULTOMIRIS (ravulizumab) or SOLIRIS (eculizumab) for the treatment of extravascular haemolysis (EVH) in adults with paroxysmal nocturnal haemoglobinuria (PNH). Danicopan has also been approved in Japan and EU for the similar indication. At present, it is being evaluated in a Phase II study for the treatment of geographic atrophy.
Note: Detailed current therapies assessment will be provided in the full report of Factor D Complement Inhibitors
Emerging Factor D inhibitor Drugs
Vemircopan: AstraZeneca/Alexion
Vemircopan is an orally bioavailable inhibitor of complement Factor D, a serine protease that cleaves complement factor B, with potential complement system inhibiting activity. Upon administration, vemircopan targets, binds to and blocks the activity of Factor D, and thereby inhibits cleavage of complement factor B into Ba and Bb in the alternative pathway of the complement cascade. This inhibits Factor D-mediated signaling and activation of the alternative complement pathway (ACP), blocks complement-mediated hemolysis in paroxysmal nocturnal hemoglobinuria (PNH) and prevents ACP-induced tissue damage.
Alexion is currently investigating vemircopan in a Phase II study for proliferative lupus nephritis (LN) or immunoglobulin A nephropathy (IgAN)
Note: Detailed emerging therapies assessment will be provided in the final report.
|
Company |
Drug |
Indication |
RoA |
Phase |
NCTID |
|
AstraZeneca/Alexion |
vemircopan |
Proliferative Lupus Nephritis (LN) or Immunoglobulin A Nephropathy (IgAN) |
Oral |
II |
NCT05097989 |
|
BioCryst |
BCX10013 |
PNH |
Oral |
I |
NCT06100900 |
Note: The emerging drug list is indicative, the full list will be given in the final report.
Factor D Complement Inhibitors Market Outlook
The launch of VOYDEYA by AstraZeneca/Alexion demonstrates that the pharma companies are putting efforts to understand the role of various complement proteins in the pathogeneis of complement mediated diseases. In addition to Factor D, the companies are also progressing with RNAi-based C5 inhibitors, Factor B inhibitors, and other approaches. Moreover, they have shifted their focus from saturated indications like PNH, aHUS, and gMG, and are now directing attention towards complement-mediated renal diseases such as IgAN and C3G. Complement inhibitors are also gaining traction in treating geographic atrophy (GA), an advanced stage of dry age-related macular degeneration (AMD), with Alexion currently assessing danacopan in a Phase II study for GA.
Factor D inhibitor Companies like BioCryst are using the PNH patient pool as a benchmark for assessing the effectiveness of their complement inhibitor. Currently, the company is investigating BCX10013 in a proof-of-concept trial involving individuals with paroxysmal nocturnal hemoglobinuria (PNH) to validate its potential. Following this, the company intends to progress into a pivotal trials targeting patients with renal complement-mediated diseases, such as IgA nephropathy (IgAN).
Factor D Inhibitors Drugs Uptake
This section focuses on the uptake rate of potential approved and emerging Complement inhibitors expected to be launched in the Factor D inhibitor market during 2020–2034.
Factor D Complement Inhibitors Pipeline Development Activities
The Factor D inhibitor drugs market report provides insights into different therapeutic candidates in Phase II, and Phase I. It also analyzes key Factor D inhibitor companies involved in developing targeted therapeutics.
The presence of numerous drugs under different stages is expected to generate immense opportunity for Factor D Complement inhibitors market growth over the forecasted period.
Recent Key events, Acquisitions and Collaborations
The Factor D inhibitor market report covers information on collaborations, acquisitions and mergers, licensing, and patent details for complement inhibitors emerging Factor D inhibitor therapies, and key development milestones.
-
Acquisition - In 2021, AstraZeneca acquired Alexion for USD 39 billion, with an aim to create a dedicated rare disease unit and enter into the market of other complement mediated disorders through Alexions’ pipeline.
-
Clinical milestones – In October 2023, BioCryst begun enrollment in a proof-of-concept clinical trial evaluating BCX10013, a potential once-daily, oral Factor D inhibitor for the treatment of PNH.
-
The goal of this proof-of-concept trial is to understand the preliminary efficacy and safety profile of once-daily dosing with BCX10013. If the results in PNH patients confirm its potential, the company plans to advance into a pivotal program in patients living with renal complement-mediated diseases, including IgA nephropathy (IgAN).
KOL Views on Factor D inhibitor
To keep up with current and future Factor D inhibitor market trends, we take Industry Experts’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry experts were contacted for insights on Factor D inhibitors' evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, drug uptake, along challenges related to accessibility.
DelveInsight’s analysts connected with 15+ KOLs to gather insights; however, interviews were conducted with 10+ KOLs in the 7MM.
Their opinion helps understand and validate current and emerging therapy treatment patterns or Factor D inhibitors market trends. This will support the clients in understanding potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
|
KOL Views |
|
“The approval of VOYDEYA offers this small subset of PNH patients an add-on therapy designed to address EVH, while maintaining disease control with Ultomiris or Soliris. Terminal complement inhibition with ULTOMIRIS can address the life-threatening complications of PNH. Dual complement pathway inhibition at Factor D and C5 may be an optimal treatment approach for this subset of patients with EVH, enabling them to continue with proven standard-of-care therapy” |
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Scope of the Factor D inhibitor Market Report
- The Factor D inhibitor market report covers a segment of key events, an executive summary, and a descriptive overview of Factor D Complement Inhibitors, explaining their mechanism, and therapies (current and emerging).
- Comprehensive insight into the Competitive Landscape, and forecasts, the future growth potential of treatment rate, drug uptake, and drug information have been provided.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current landscape.
- A detailed review of the Factor D Complement Inhibitors market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The Factor D inhibitor market report provides an edge while developing business strategies, by understanding trends, through SWOT analysis, expert insights/KOL views, and treatment preferences that help shape and drive the 7MM Factor D Complement inhibitors market.
Factor D Complement Inhibitors Market Report Insights
- Factor D Complement Inhibitors Targeted Patient Pool
- Therapeutic Approaches
- Factor D Inhibitors Pipeline Analysis
- Factor D Inhibitors Market Size
- Factor D inhibitor Market Trends
- Existing and Future Factor D inhibitor Market Opportunity
Factor D inhibitors Market Report Key Strengths
- Eleven-years Forecast
- The 7MM Coverage
- Key Cross Competition
- Factor D inhibitor Drugs Uptake
- Key Factor D inhibitor Market Forecast Assumptions
Factor D Inhibitors Market Report Assessment
- Current Treatment Practices
- Factor D inhibitor Unmet Needs
- Factor D inhibitor Pipeline Product Profiles
- Factor D inhibitor Market Attractiveness
- Qualitative Analysis (SWOT)
Key Questions
- What was the Factor D Complement Inhibitor market size, the market size by therapies, market share (%) distribution, and what would it look like in 2034? What are the contributing factors for this growth?
- Which Factor D inhibitor drug is going to be the largest contributor in 2034?
- Which is the most lucrative market for Factor D Complement Inhibitors?
- Which Factor D inhibitor type segment accounts for maximum Factor D Complement Inhibitor sales?
- What are the pricing variations among different geographies for approved therapies?
- How has the reimbursement landscape for Factor D Complement Inhibitors evolved since the first one was approved? Do patients have any access issues that are driven by reimbursement decisions?
- What are the risks, burdens, and unmet needs of treatment with Factor D Complement Inhibitors? What will be the growth opportunities across the 7MM for the patient population of Factor D Complement Inhibitors?
- What are the key factors hampering the growth of the Factor D Complement Inhibitors market?
- What are the indications for which recent novel therapies and technologies have been developed to overcome the limitations of existing treatments?
- What key designations have been granted to the therapies for Factor D Complement Inhibitors?
- What is the cost burden of approved Factor D inhibitor therapies on the patient?
- Patient acceptability in terms of preferred therapy options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved Factor D inhibitor therapies?
Reasons to buy
- The Factor D inhibitor market report will help develop business strategies by understanding the latest trends and changing dynamics driving the Factor D Complement Inhibitors Market.
- Understand the existing Factor D inhibitor market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain) the United Kingdom, and Japan.
- Identifying strong upcoming Factor D inhibitor companies in the Factor D inhibitor market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of indication-wise current and emerging Factor D inhibitor therapies under the conjoint analysis section to provide visibility around leading indications.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing Factor D inhibitor market so that the upcoming Factor D inhibitor companies can strengthen their development and launch strategy.