Papulopustular Rosacea Market
- The global Papulopustular Rosacea treatment market is expected to grow significantly in the coming years during the forecast period (2024-2034) driven by the rising prevalence of rosacea, particularly among geriatric patients, the raised awareness about rosacea among both patients and healthcare professionals contribute to its early detection and treatment initiation, and the growing emphasis on individualized therapy.
- Papulopustular Rosacea can significantly impact patients' quality of life due to its visible symptoms and potential psychosocial effects. As a result, there is growing emphasis on developing treatments that not only address the physical symptoms but also improve overall well-being and self-esteem.
- The approved therapies in the global Rosacea treatment market include key players such as CollaGenex Pharmaceuticals, Sol-Gel Technologies, Galderma, and others
- Several emerging drugs for Papulopustular Rosacea are in clinical phase development, aiming to address its inflammatory nature including TP-04 (Tarsus Pharmaceuticals), PF-07038124 (Pfizer), and others. These include novel topical formulations targeting specific inflammatory pathways, as well as systemic medications with improved efficacy and safety profiles.
To know more in detail about the Papulopustular Rosacea Market Dynamics, click here @ Papulopustular Rosacea Market Report
DelveInsight’s comprehensive report titled “Papulopustular Rosacea Market Insights, Epidemiology, and Market Forecast – 2034” offers a detailed analysis of Papulopustular Rosacea. The report presents historical and projected epidemiological data covering total prevalent cases of rosacea, diagnosed prevalent cases of rosacea, diagnosed prevalent cases of papulopustular rosacea, and treated cases of papulopustular rosacea. In addition to epidemiology, the Papulopustular Rosacea market report encompasses various aspects related to the patient population. These aspects include the diagnosis process, prescription patterns, physician perspectives, market accessibility, treatment options, and prospective developments in the market across seven major markets: the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan, spanning from 2020 to 2034.
The Papulopustular Rosacea market report analyzes the existing treatment practices and unmet medical requirements in Papulopustular Rosacea. It evaluates the market potential and identifies potential business prospects for enhancing therapies or interventions. This valuable information enables stakeholders to make well-informed decisions regarding product development and strategic planning for the market.
Papulopustular Rosacea Treatment Market
Papulopustular Rosacea Overview
Rosacea is a common skin condition characterized by facial redness or erythema, affecting approximately one in ten people globally. It encompasses four main types: erythematotelangiectatic, papulopustular, phymatous, and ocular rosacea. Papulopustular rosacea, a subtype of rosacea, is associated with “whitehead” pustules, which are pus-filled blemishes, and red, swollen bumps. These typically appear on the cheeks, chin, and forehead and are frequently misidentified as acne. These signs and symptoms may flare up for a period of weeks to months and then diminish for a while. The disease sometimes can be mistaken for acne (an allergic reaction or other skin problems). Moreover, these symptoms often appear in intermittent flare-ups and can be triggered by factors like genetics, immune system irregularities, and environmental stimuli such as sunlight, stress, and certain foods.
Papulopustular Rosacea Diagnosis and Treatment Algorithm
The Papulopustular Rosacea diagnosis is based on a compatible history and physical examination. The patient may be misdiagnosed with skin conditions that share similar features as adult acne vulgaris, photodermatitis, seborrheic dermatitis, or contact dermatitis.
The current treatment procedures for Papulopustular Rosacea include topical drugs such as metronidazole, azelaic acid, ivermectin, and dapsone as first-line options. Severe cases may require oral antibiotics such as doxycycline and isotretinoin. Combination therapy may be indicated, attempting to manage symptoms, reduce inflammation, and prevent flare-ups.
Papulopustular Rosacea Epidemiology
The epidemiology section on the Papulopustular Rosacea market report offers information on the patient populations, including historical and projected trends for each of the seven major markets. Examining key opinion leader views from physicians or clinical experts can assist in identifying the reasons behind historical and projected trends. The prevalence patient pool, their trends, and the underlying assumptions are all included in this section of the report.
This section also presents the data with relevant tables and graphs, offering a clear and concise view of the prevalence of Papulopustular Rosacea. Additionally, the report discloses the assumptions made during the analysis, ensuring data interpretation and presentation transparency. This epidemiological data is valuable for understanding the disease burden and its impact on the patient population across various regions.
Key Findings
- According to the National Rosacea Society, approximately 415 million individuals are affected with Rosacea globally, out of which nearly 16 million people are reported in the US. Within the US, the occurrence of rosacea appears to be increasing.
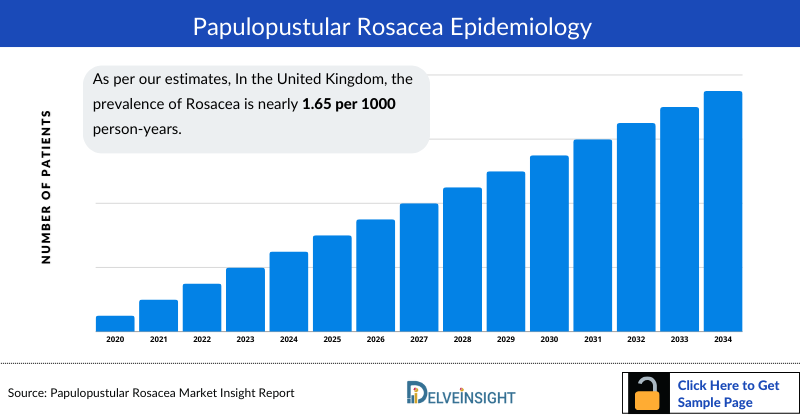
- As per our estimates, In the United Kingdom, the prevalence of Rosacea is nearly 1.65 per 1000 person-years. There is a paucity of epidemiological data on Rosacea, with reported prevalence rates ranging from as little as 0.09% to as much as 22.00%.
- In a study examining the epidemiology of rosacea in the German population, it was noted that among a cohort of 90,880 office workers, there was a prevalence rate of 2.3%.
- It is estimated that females are more susceptible to rosacea compared to males with nearly 87% of cases occurring in females and 13% in males in Germany, based on studies involving clinical assessments by dermatologists and demographic surveys. Additionally, among patients diagnosed with rosacea, 64% had erythematotelangiectatic subtype, 36% had a papulopustular subtype, 24% had phymatous subtype, and 36% had an ocular subtype. Among those experiencing at least two subtypes, the majority (66%) developed the erythematotelangiectatic subtype before the papulopustular subtype, 92% developed the erythematotelangiectatic subtype before phymatous subtype, and 83% developed papulopustular subtype before phymatous subtype.
Papulopustular Rosacea Market Outlook
The treatment goal for Papulopustular Rosacea is to reduce inflammation, manage papules and pustules, reduce redness, and prevent flare-ups with topical and oral medications. Current treatment options include topical medications like metronidazole, azelaic acid, ivermectin, and sulfacetamide-sulfur, which aim to reduce inflammation and address symptoms such as papules and pustules. Additionally, oral antibiotics like doxycycline and minocycline, often used in combination therapies, target bacterial overgrowth and inflammation associated with the condition. Procedures like intense pulsed light therapy or laser therapy can also be utilized to target visible blood vessels and diminish redness linked with Papulopustular Rosacea.
The emerging Papulopustular Rosacea market for developing medications in Papulopustular Rosacea is rising, with an emphasis on increasing therapy options. New medicines, such as TP-04, and GABA-A receptor antagonists, have the potential to help manage symptoms. Thus, with ongoing research and continued dedication, the future holds hope for even more effective treatments and, ultimately, a cure for this challenging condition.
According to DelveInsight, the Papulopustular Rosacea market in the 7MM is expected to change significantly by 2034.
Recent Developments in Papulopustular Rosacea Clinical Trials:
- On November 5, 2024, the FDA approved minocycline hydrochloride extended-release capsules (DFD-29, Emrosi; Journey Medical) for treating inflammatory lesions caused by rosacea in adults. The approval is based on positive results from two phase 3 clinical trials, MVOR-1 (NCT05296629) and MVOR-2 (NCT05343455), which evaluated DFD-29 in patients aged 18 and older with rosacea.
Papulopustular Rosacea Drug Chapters
Marketed Papulopustular Rosacea Drugs
EPSOLAY (benzoyl peroxide): Sol-Gel Technologies/ Galderma
In April 2022, the US FDA approved EPSOLAY, an innovative topical cream containing encapsulated benzoyl peroxide, 5%, for the treatment of inflammatory lesions of rosacea (papulopustular rosacea or PPR) in adult patients. The product uses Sol Gel’s patented microencapsulation technology that creates a barrier between the skin and the dose, and acts as a keratinocyte and protein synthesis inhibitor. The technology aims to reduce the risk of benzoyl peroxide’s oxidative effect which can cause skin irritation. It.
ORACEA (doxycycline): CollaGenex Pharmaceuticals
In May 2006, the US FDA approved ORACEA (doxycycline), a once-a-day capsule formulation of doxycycline, consisting of a combination of immediate and delayed-release beads indicated for the treatment of inflammatory lesions (papules and pustules) of Rosacea in adult patients. It is scientifically designed to deliver a timed-release dose of doxycycline that safely keeps patients below the antibiotic threshold while providing sustained anti-inflammatory effects throughout the day. It acts as a protein 30S ribosomal subunit inhibitor.
Note: Detailed marketed therapies assessment will be provided in the final report...
Emerging Papulopustular Rosacea Drugs
The emerging pipeline for the treatment of Papulopustular Rosacea has few candidates. These products are in the mid or last stage of investigation. With the launch of emerging therapies, the total market of Papulopustular Rosacea is supposed to experience immense changes in the future. Some of the drugs in the pipeline include TP-04 (Tarsus Pharmaceutical), PF-07038124 (Pfizer), and others.
TP-04: Tarsus Pharmaceuticals
TP-04, developed by Tarsus Pharmaceutical, is an investigational, topical, aqueous gel formulation of lotilaner in development for the potential treatment of Papulopustular Rosacea. The drug candidate completed a Phase IIa (Galatea) clinical trial, demonstrating statistically significant improvements in inflammatory lesions and IGA score compared to the placebo, indicating TP-04 was generally well tolerated with no serious adverse events. Furthermore, as per the latest report, the company has planned a US FDA meeting in 2024.
PF-07038124: Pfizer
PF-07038124 is a novel molecular entity that works by targeting phosphodiesterase 4 (PDE4). It is being developed as a topical ointment to treat Papulopustular Rosacea. Currently, the drug candidate is being investigated in a Phase II clinical trial for Papulopustular Rosacea, expected to be completed by December 2024. Additionally, PF-07038124 is also being investigated for treating atopic dermatitis and psoriasis.
Note: Detailed emerging therapies assessment will be provided in the final report...
Papulopustular Rosacea Market Segmentation
DelveInsight’s ‘Papulopustular Rosacea Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a detailed outlook of the current and future Papulopustular Rosacea market, segmented within countries, by therapies, and by classes. Further, the Papulopustular Rosacea market of each region is then segmented by each therapy to provide a detailed view of the current and future Papulopustular Rosacea market share of all therapies.
Papulopustular Rosacea Market Size by Countries
The Papulopustular Rosacea market size is assessed separately for various countries, including the United States, EU4 (Germany, France, Italy, and Spain), the UK, and Japan. In 2023, the United States held a significant share of the overall 7MM (Seven Major Markets) Papulopustular Rosacea market, primarily attributed to the country's higher prevalence of the condition and the elevated cost of the available treatments. This dominance is projected to persist, especially with the potential early introduction of new products.
Papulopustular Rosacea Market Size by Therapies
Papulopustular Rosacea Market Size by Therapies is categorized into current and emerging markets for the study period 2020–2034. One of the emerging Papulopustular Rosacea drugs anticipated to launch during the forecast period is TP-04, a lotilaner API that has shown strong efficacy in multiple studies for eliminating demodex mites, which are commonly found in the skin of individuals with papulopustular rosacea and might play a role in the inflammation linked to the condition.
Note: Detailed market segment assessment will be provided in the final report...
Papulopustular Rosacea Drugs Uptake
This section focuses on the sales uptake of potential Papulopustular Rosacea drugs that have recently been launched or are anticipated to be launched in the Papulopustular Rosacea market between 2020 and 2034. It estimates the market penetration of Papulopustular Rosacea drugs for a given country, examining their impact within and across classes and segments. It also touches upon the financial and regulatory decisions contributing to the probability of success (PoS) of the drugs in the Papulopustular Rosacea market.
The emerging Papulopustular Rosacea therapies are analyzed based on various attributes such as safety and efficacy in randomized clinical trials, order of entry and other market dynamics, and the unmet need they fulfill in the Papulopustular Rosacea market.
Note: Detailed assessment of drug uptake and attribute analysis will be provided in the full report on Papulopustular Rosacea...
Papulopustular Rosacea Market Access and Reimbursement
DelveInsight’s ‘Papulopustular Rosacea Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a descriptive overview of the market access and reimbursement scenario of Papulopustular Rosacea.
This section includes a detailed analysis of the country-wise healthcare system for each therapy, enlightening the market access, reimbursement policies, and health technology assessments.
Papulopustular Rosacea KOL Views
To keep up with current Papulopustular Rosacea market trends and fill gaps in secondary findings, we interview KOLs and SMEs’ working in the Papulopustular Rosacea domain. Their opinion helps understand and validate current and emerging therapies and treatment patterns or Papulopustular Rosacea market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the Papulopustular Rosacea unmet needs.
Papulopustular Rosacea: KOL Insights
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. These KOLs were from organizations, institutes, and hospitals such as Johns Hopkins University School of Medicine in the US, Mount Sinai Hospital in the US, Goethe University Hospital in Germany, and Henri Mondor Hospital in France, among others.
“While existing treatments like topical and oral medications can alleviate symptoms, they may not provide complete resolution for all patients, leaving room for more effective therapeutic options. Therefore, both healthcare providers and patients are eagerly anticipating the approval of upcoming therapies.”
“Papulopustular Rosacea shares clinical features with other skin conditions such as acne vulgaris and seborrheic dermatitis, leading to diagnostic confusion, especially in milder cases or when multiple conditions coexist.”
Note: Detailed assessment of KOL Views will be provided in the full report on Papulopustular Rosacea...
Competitive Intelligence Analysis
We conduct a Competitive and Market Intelligence analysis of the Papulopustular Rosacea Market, utilizing various Competitive Intelligence tools such as SWOT analysis and Market entry strategies. The inclusion of these analyses is contingent upon data availability, ensuring a comprehensive and well-informed assessment of the market landscape and competitive dynamics.
Papulopustular Rosacea Pipeline Development Activities
The Papulopustular Rosacea market report offers an analysis of therapeutic candidates in Phase II and III stages and examines companies involved in developing targeted therapeutics for Papulopustular Rosacea. It provides valuable insights into the advancements and progress of potential treatments in clinical development for this condition.
Pipeline Development Activities
The Papulopustular Rosacea market report covers information on collaborations, acquisitions and mergers, licensing, patent details, and other information for emerging Papulopustular Rosacea therapies.
Papulopustular Rosacea Market Report Insights
- Papulopustular Rosacea Patient Population
- Therapeutic Approaches
- Papulopustular Rosacea Pipeline Analysis
- Papulopustular Rosacea Market Size
- Papulopustular Rosacea Market Trends
- Papulopustular Rosacea Market Opportunities
- Impact of Upcoming Papulopustular Rosacea Therapies
Papulopustular Rosacea Market Report Key Strengths
- 11 Years Forecast
- The 7MM Coverage
- Papulopustular Rosacea Epidemiology Segmentation
- Key Cross Competition
- Highly Analyzed Papulopustular Rosacea Market
- Papulopustular Rosacea Drugs Uptake
Papulopustular Rosacea Market Report Assessment
- Papulopustular Rosacea Current Treatment Practices
- Unmet Needs
- Papulopustular Rosacea Pipeline Product Profiles
- Papulopustular Rosacea Market Attractiveness
Key Questions
- How common is Papulopustular Rosacea?
- What are the key findings of Papulopustular Rosacea epidemiology across the 7MM, and which country will have the highest number of patients during the study period (2020–2034)?
- What are the currently available treatments for Papulopustular Rosacea?
- What are the disease risk, burden, and unmet needs of Papulopustular Rosacea?
- At what CAGR is the Papulopustular Rosacea market and its epidemiology is expected to grow in the 7MM during the forecast period (2024–2034)?
- How would the unmet needs impact the Papulopustular Rosacea market dynamics and subsequently influence the analysis of the related trends?
- What would be the forecasted patient pool of Papulopustular Rosacea in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), the UK, and Japan?
- Among EU4 and the UK, which country will have the highest number of patients during the forecast period (2024–2034)?
- How many Papulopustular Rosacea companies are currently developing therapies for the treatment of Papulopustular Rosacea?
Frequently Asked Questions
1. Is Papulopustular Rosacea contagious?
No, Papulopustular Rosaceais not contagious. It's a chronic inflammatory skin condition, not an infectious disease.
2. Can Papulopustular Rosacea affect other parts of the body besides the face?
While Rosacea primarily affects the face, it can occasionally involve the eyes (ocular Rosacea) or other areas of the body. However, Papulopustular Rosacea mainly affects the central face.
3. Are there any clinical trials for Papulopustular Rosacea treatments?
Research and clinical trials are ongoing to explore potential treatments for Papulopustular Rosacea. Some experimental treatments, such as fibroblast growth factor stimulants, have shown promise in slowing disease progression.
4. How is the market size estimated in the forecast report?
The market size is estimated through data analysis, statistical modeling, and expert opinions. It may consider factors such as prevalence cases, treatment costs, revenue generated, and market trends.
5. Is there an analysis of the market’s competitive landscape in the report?
The market forecast report will likely offer insights into key market players, their product offerings, partnerships, and strategies, providing stakeholders with a comprehensive understanding of the competitive dynamics in the Papulopustular Rosacea market.