Pediatric Obesity Market
Key Highlights
- The Pediatric Obesity market size is anticipated to grow with a significant CAGR duting the study period (2020-2034).
- Obesity is the most common nutritional disorder among children and adolescents, now seen as a major health concern in the developed world due to its rapid rise. The National Health and Nutrition Examination Survey (NHANES) shows increasing obesity rates across all pediatric age groups and sexes. Factors such as genetics, environment, metabolism, lifestyle, and eating habits contribute to this complex disorder.
- In 2023, there were approximately 8 million prevalent cases of obesity among children aged 5 to 19 in the 7MM. Given current lifestyle trends, this number is expected to increase at a significant rate.
- The available treatments for pediatric obesity include a comprehensive approach that combines lifestyle modifications, behavioral therapy, and medication. Lifestyle interventions involve changes to diet and physical activity. The currently available therapy includes XENICAL, SAXENDA, WEGOVY, IMCIVREE, and others.
- Among the 7MM, the US accounts for the highest market share of pediatric obesity.
- Recently, significant advancements have been made in the treatment landscape for pediatric obesity. For instance, in March 2024, the US FDA approved a new indication for WEGOVY (semaglutide) injection, which is now approved to reduce the risk of cardiovascular death, heart attack, and stroke in adults with cardiovascular disease who are either obese or overweight.
- Rhythm Pharmaceuticals plans to submit a supplemental New Drug Application (sNDA) to the US FDA in the second quarter of 2024, seeking to expand the label for IMCIVREE to include children aged 2 to 6 years.
- However, there is a critical need for a stronger pipeline of emerging therapies, for pediatric patients suffering from obesity, as current developments are insufficient to meet the growing demand.
DelveInsight's “Pediatric Obesity Market Insights, Epidemiology and Market Forecast – 2034” report delivers an in-depth understanding of pediatric obesity, historical and forecasted epidemiology as well as the pediatric obesity therapeutics market trends in the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan.
The pediatric obesity market report provides real-world prescription pattern analysis, emerging drugs, market share of individual therapies, and historical and forecasted 7MM pediatric obesity market size from 2020 to 2034. The report also covers current pediatric obesity treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s underlying potential.
| Study Period | 2020–2034 |
| Forecast Period | 2024–2034 |
| Geographies Covered | US, EU4 (Germany, France, Italy, and Spain) the UK, and Japan |
| Pediatric Obesity Epidemiology
| Segmented by: Total Prevalent Cases of Obesity in Children Total Obesity patients seeking help in Children Total Treated Cases of Obesity in Children |
| Pediatric Obesity key companies | Novo Nordisk Rhythm Pharmaceuticals |
| Pediatric Obesity key therapies/drug | XENICAL SAXENDA WEGOVY IMCIVREE |
| Pediatric Obesity Market | Segmented by: · Region · Therapies |
| Analysis | · KOL Views · SWOT Analysis · Reimbursement · Conjoint Analysis · Unmet needs |
Pediatric Obesity Treatment Market
Pediatric Obesity Overview, Country-Specific Treatment Guidelines and Diagnosis
Obesity is a complex, pervasive, and frequently persistent health problem in children and adolescents. Pediatric obesity is commonly defined using body mass index, a ratio of weight to squared height. In the US, the CDC defines pediatric obesity as a BMI at or above the 95th percentile and severe obesity as above 120% of the 95th percentile (or over 35 kg/m²) based on age- and sex-specific growth charts. Pediatric obesity is a significant public health concern that requires a comprehensive approach to prevention and treatment. Early identification and intervention are crucial to prevent the long-term complications associated with obesity in children.
Pediatricians assess various aspects of a child's weight status due to the considerable variation in growth trends and body frames among children. These assessments include growth charts, family history of obesity, eating habits, activity levels, and psychosocial history, which encompasses sleep patterns, mood disorders like depression, social interactions, and factors such as bullying, as well as other health conditions. When a child is suspected of being overweight, pediatricians may order lab tests such as cholesterol tests, blood sugar tests, and blood tests to check for hormone imbalances and obesity-linked conditions.
Other Types of Obesity:
1. Obesity – A chronic condition characterized by excessive fat accumulation, leading to metabolic disorders such as diabetes and cardiovascular diseases. It is influenced by genetic, lifestyle, and environmental factors.
2. Hypothalamic Obesity – Caused by damage to the hypothalamus, the brain's hunger and metabolism control center, leading to unregulated appetite, low energy expenditure, and rapid weight gain.
3. HET Obesity/POMC Deficiency Obesity – A rare genetic disorder resulting from mutations in the Pro-Opiomelanocortin (POMC) gene, causing severe early-onset obesity due to impaired appetite regulation.
4. Pro-Opiomelanocortin (POMC) Deficiency Obesity & Leptin Receptor (LEPR) Deficiency Obesity – Genetic conditions that disrupt appetite control, leading to excessive hunger and severe obesity from an early age.
5. Syndromic and Monogenic Obesity – Rare obesity types caused by single-gene mutations or syndromic conditions (e.g., Prader-Willi Syndrome), often associated with developmental and metabolic abnormalities.
The pediatric obesity report provides an overview of pediatric obesity pathophysiology, diagnostic approaches, and detailed treatment algorithm along with a real-world scenario of a patient’s journey beginning from the first symptom, the time taken for diagnosis to the entire treatment process.
Further details related to country-based variations in diagnosis are provided in the report...
Pediatric Obesity Treatment
Overweight and obesity, as well as their related non-communicable diseases, are largely preventable and manageable. At the individual level, people may be able to reduce their risk by adopting preventive interventions at each step of the life cycle, starting from pre-conception and continuing during the early years. Pediatric obesity treatment involves a comprehensive approach that includes lifestyle changes, medications, and in some cases, weight-loss surgery. For adolescents aged 12 years and older with obesity, the FDA has approved several medications to aid in weight management, including XENICAL, SAXENDA, and WEGOVY. These medications are used in conjunction with lifestyle modifications to help achieve clinically significant weight loss. Early recognition and intervention are crucial to prevent long-term complications and improve overall health.
Pediatric Obesity Epidemiology
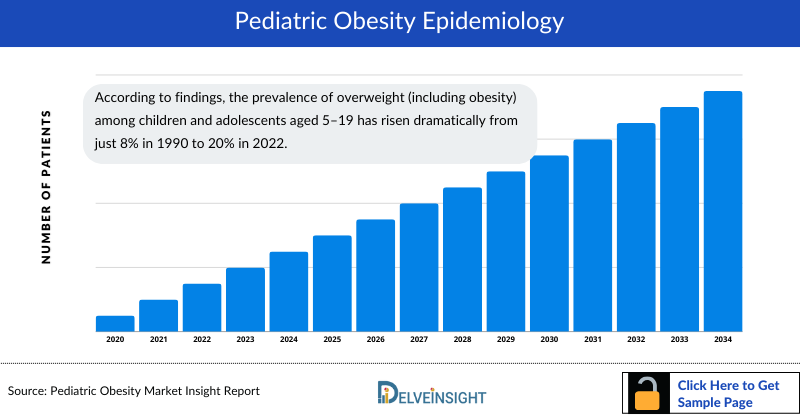
The pediatric obesity epidemiology chapter in the report provides historical as well as forecasted in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain), the United Kingdom, and Japan from 2024 to 2034. Pediatric obesity epidemiology is segmented with detailed insights into Total Prevalent Cases of Obesity in Children, Total Obesity Patients Seeking help in Children, and Total Treated Cases of Obesity in Children.
- According to findings, the prevalence of overweight (including obesity) among children and adolescents aged 5–19 has risen dramatically from just 8% in 1990 to 20% in 2022. The rise has occurred similarly among both boys and girls: in 2022 19% of girls and 21% of boys were overweight.
- Among EU4 and the UK, Germany accounted for the highest number of prevalent cases of obesity in children whereas Spain accounted for the lowest number of cases.
Pediatric Obesity Drug Chapters
The drug chapter segment of the pediatric obesity report encloses a detailed analysis of pediatric obesity marketed drugs and pipeline drugs. It also deep dives into the pediatric obesity pivotal clinical trial details; recent and expected market approvals, patent details, the latest news, and recent deals and collaborations.
Pediatric Obesity Marketed Drugs
WEGOVY (semaglutide): Novo Nordisk
WEGOVY is a GLP-1 receptor agonist indicated for use with a reduced calorie diet and increased physical activity. It helps reduce the risk of major cardiovascular events, such as cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke, in adults with established cardiovascular disease who are obese or overweight. Additionally, WEGOVY aids in long-term weight reduction for adults and pediatric patients aged 12 years and older with obesity, as well as adults with overweight who have at least one weight-related comorbid condition.
SAXENDA (liraglutide): Novo Nordisk
SAXENDA is a GLP-1 receptor agonist indicated as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management. It is prescribed for adults with a BMI of 30 kg/m² or greater (obese) or 27 kg/m² or greater (overweight) with at least one weight-related comorbid condition, such as hypertension, type 2 diabetes, or dyslipidemia. It is also indicated for pediatric patients aged 12 years and older who weigh over 60 kg and have a BMI corresponding to 30 kg/m² for adults by international cut-offs.
IMCIVREE (setmelanotide): Rhythm Pharmaceuticals
IMCIVREE is a melanocortin 4 (MC4) receptor agonist indicated for chronic weight management in adult and pediatric patients aged 6 years and older with monogenic or syndromic obesity. It is used specifically for those with obesity due to Pro-opiomelanocortin (POMC), proprotein convertase subtilisin/kexin type 1 (PCSK1), or leptin receptor (LEPR) deficiency, as determined by an FDA-approved test showing pathogenic, likely pathogenic, or uncertain significance (VUS) variants in the POMC, PCSK1, or LEPR genes. IMCIVREE is also indicated for patients with Bardet-Biedl syndrome (BBS).
| Therapy Name | Company Name | ROA | MOA | Any Special Status |
| WEGOVY | Novo Nordisk | Subcutaneous | GLP-1 receptor agonist | Priority Review Designation |
| SAXENDA | Novo Nordisk | Subcutaneous | GLP-1 receptor agonist | NA |
| IMCIVREE | Rhythm Pharmaceuticals | Subcutaneous | MC4 receptor agonist | BTD/ ODD (POMC-deficiency obesity) |
Note: Detailed current therapies assessment will be provided in the full report of pediatric obesity...
Emerging Pediatric Obesity Drugs
Tirzepatide: Eli Lilly and Company
LY3298176 (GIP/GLP-1 Receptor Agonist) is a biologic entity that acts as an agonist of both the glucose-dependent insulinotropic polypeptide (GIP) receptor and the glucagon-like peptide-1 (GLP-1) receptor and is being studied for the reduction of morbidity and mortality in Obesity. The company has recently initiated a Phase I study of tirzepatide for treating pediatric participants aged 6 to 11 years with obesity. Under the brand name ZEPBOUND, tirzepatide is already approved as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adults. It is indicated for adults with an initial BMI of 30 kg/m² or greater (obesity) or 27 kg/m² or greater (overweight) who have at least one weight-related comorbid condition, such as hypertension, dyslipidemia, type II diabetes mellitus, obstructive sleep apnea, or cardiovascular disease.
Note: Detailed emerging therapies assessment will be provided in the final report...
Pediatric Obesity Market Outlook
Key players, such as Eli Lilly and Company, and others are evaluating their candidates in different stages of clinical development. They aim to investigate their products for the treatment of pediatric obesity.
- The United States accounts for the largest market size of pediatric obesity, in comparison to EU4 (Germany, Spain, Italy, France), the United Kingdom, and Japan.
- Among EU4 and the UK, Germany had the highest market size.
Pediatric Obesity Drugs Uptake
This section focuses on the uptake rate of potential drugs expected to be launched in the market during 2024–2034, which depends on the competitive landscape, safety, and efficacy data along with order of entry. It is important to understand that the key players evaluating their novel therapies in the pivotal and confirmatory trials should remain vigilant when selecting appropriate comparators to stand the greatest chance of a positive opinion from regulatory bodies, leading to approval, smooth launch, and rapid uptake.
Further detailed analysis of emerging therapies drug uptake in the report…
Pediatric Obesity Activities
The report provides insights into Pediatric Obesity clinical trials within in Phase I, Phase II, and Phase III stages. It also analyzes key players involved in developing targeted therapeutics.
Pipeline Development Activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for pediatric obesity emerging therapies.
KOL Views
To keep up with the real-world scenario in current and emerging market trends, we take opinions from Key Industry leaders working in the domain through primary research to fill the data gaps and validate our secondary research. Industry experts were contacted for insights on the evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility.
DelveInsight’s analysts connected with 10+ KOLs to gather insights; however, interviews were conducted with 5+ KOLs in the 7MM. Their opinion helps understand and validate current and emerging treatment patterns of obesity. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
| Region | KOL Views |
| United States | “To determine if somebody is obese, body mass index is considered, a measure of body fat based on height and weight. The normal range for BMI is 18 to 25. A BMI over 25 is considered overweight, while if somebody has a BMI over 30, that is considered obese, and they are at risk for developing health problems due to their weight. Obesity negatively affects every organ system in the body, therefore, many health issues are directly affected by obesity.” |
| UK | “People tend to become overweight because they regularly consume more calories than they expend. A lot has to do with the quality of the diet and the various habits that people can get into, such as snacking regularly.” |
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in event-free survival, one of the most important primary outcome measures is event-free survival and overall survival.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Market Access and Reimbursement
The report provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of currently used therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Pediatric Obesity Report
- The report covers a segment of key events, an executive summary, descriptive overview of pediatric obesity, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, and disease progression along with country specific treatment guidelines.
- Additionally, an all-inclusive account of both the current and emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will have an impact on the current treatment landscape.
- A detailed review of the pediatric obesity market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM pediatric obesity market.
Pediatric Obesity Report Insights
- Pediatric Obesity Patient Population
- Pediatric Obesity Therapeutic Approaches
- Pediatric Obesity Pipeline Analysis
- Pediatric Obesity Market Size and Trends
- Existing and future Market Opportunity
Pediatric Obesity Report Key Strengths
- Eleven Years Forecast
- 7MM Coverage
- Pediatric Obesity Epidemiology Segmentation
- Inclusion of Country specific treatment guidelines
- KOL’s feedback on approved and emerging therapies
- Key Cross Competition
- Conjoint analysis
- Pediatric Obesity Drugs Uptake
- Key Pediatric Obesity Market Forecast Assumptions
Pediatric Obesity Report Assessment
- Current Pediatric Obesity Treatment Practices
- Pediatric Obesity Unmet Needs
- Pediatric Obesity Pipeline Product Profiles
- Pediatric Obesity Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
- Pediatric Obesity Market Drivers
- Pediatric Obesity Market Barriers
FAQs
- What is the growth rate of the 7MM pediatric obesity treatment market?
- What was the pediatric obesity total market size, the market size by therapies, market share (%) distribution in 2020, and what would it look like in 2034? What are the contributing factors/key catalysts for this growth?
- Is there any unexplored patient setting that can open the window for growth in the future?
- What are the pricing variations among different geographies for approved and off-label therapies?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends?
- What are the current and emerging options for the treatment of pediatric obesity?
- How many companies are developing therapies for the treatment of pediatric obesity?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- Patient/physician acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies?
Reasons to buy Pediatric Obesity Market Forecast Report
- The report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the pediatric obesity market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying strong upcoming players in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.